Journal of Neurology, Neurological Science and Disorders
Peculiarities of spinal epidural abscess in COVID-19 patients: a literature review
Pietro Domenico Giorgi1, Giuseppe Rosario Schirò1, Davide Colistra2, Simona Legrenzi1, Jacopo Falco2, Maria Ludovica Pallotta3* and Giuseppe Talamonti2
2Neurosurgery Unit, Emergency and Urgency Department, A.S.S.T. Grande Ospedale Metropolitano Niguarda, Milan, Italy.
3Orthopedics and Traumatology Residency in Università degli Studi Di Milano, Milan, Italy
Cite this as
Giorgi PD, Schirò GR, Colistra D, Legrenzi S, Falco J, et al (2023) Peculiarities of spinal epidural abscess in COVID-19 patients: a literature review. J Neurol Neurol Sci Disord 9(1): 004-008. DOI: 10.17352/jnnsd.000050Copyright
© 2023 Giorgi PD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.During the SARS-CoV-2 pandemic, some authors described an increased incidence of primary Spinal Epidural Abscess (SEA) in COVID-19 patients with clinical and radiological peculiarities. Early recognition of this disease remains challenging, resulting in delayed diagnosis and significant morbidity and mortality. The authors performed a systematic review of the literature in PubMed, Cochrane, and Scopus about the traditional form of SEA comparing the main features related to COVID-19 SEA. The search was performed from 1990 to 2020. Magnetic Resonance Imaging with contrast is mandatory to recognize this kind of lesion in order to obtain a differential diagnosis. COVID-19 SEAs are generally active abscesses, with a large cystic component. Gadolinium-enhanced MR images can aid in the definition of the age and consistency of the abscess. A rim of tissue that enhances after the injection of gadolinium represents granulation tissue; liquid pus is associated with an area of low signal intensity on T1-weighted images.
This new type of SEA in COVID-19 patients showed several differences also in clinical features. No evidence of an external infective source was found (spondylodiscitis, surgery, or percutaneous treatment). None of the patients was a drug abuser and MSSA was the only responsible pathogen. The cervicothoracic spine was the most involved site. Further studies are needed to confirm these preliminary findings.
Introduction
Spinal Epidural Abscess (SEA) is a rare infection of the spine [1], consisting of an accumulation of purulent fluid in the epidural space. The major risk factor of SEA is the active use of the intravenous drug (IVDU), followed by immunodeficiency, diabetes, obesity, and previous spinal surgery [2]. Within the spinal epidural abscesses (SEAs), there is a differentiation between SEAs related to pyogenic infectious spondylodiscitis and SEAs without neuroradiological signs of vertebral or disc infection. It is estimated that approximately 37% of patients with spondylodiscitis will develop an epidural abscess. On the other hand, primary SEAs not associated with spondylodiscitis are about 13.79% to 35.4% of all SEAs [3-5]. In fact, primary or spontaneous SEA is even rarer [1] and its recognition remains challenging due to the non-specificity of initial signs and symptoms, and several differential diagnoses [2,3]. This leads to a delayed diagnosis, with significant morbidity and mortality [6,7]. During the pandemic of SARS-CoV-2, a group in the Milan metropolitan area described an increase in the incidence of primary SEA in COVID-19 patients, not related to spondylodiscitis, with clinical and radiological peculiarities [8]. The aim of this review is to show the peculiarity of Magnetic Resonance Imaging (MRI) in primary SEA and, specifically, in COVID-19 patients. The importance of early diagnosis and treatment, reducing the significant morbidity and mortality associated, is crucial.
Materials and methods
A systematic analysis of the literature was performed using PubMed, Cochrane, and Scopus, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement. Studies published from January, 1st 1990 to October, 31st 2020, in English language or at least with an English abstract, were considered for initial review. First of all, we used sequential keywords (“spinal epidural abscess”; “abscess COVID-19”), and then checked for other possibly relevant articles in references. We included clinical studies, clinical trials, observational studies, case reports, and reviews with new surgical records. We chose to exclude letters to the editor, pure reviews without new surgical records, surveys, editorials, and clinical images. A literature search was first conducted using the words “spinal epidural abscess” and “abscess COVID-19” and we found 634 articles (Figure 1). We excluded 55 studies because duplicated. Then, according to the inclusion and exclusion criteria, 98 studies have been excluded. Finally, as a result of our systematic analysis, we considered 481 articles (Figure 2).
Evidence synthesis of SEA
SEA is a quite rare condition, with a low incidence ranging from 0.2 to 2.8 per 10.000 hospital admissions [1,9-11]. Recent studies reported a mortality rate of 3.7% – 5% [6,7]. Moreover, about one-third of survivors have poor neurological outcomes [2,12,13]. The average age of patients is 63 years and the ratio of men to women is 4:1 [1,2,11]. The most relevant risk factor is IVDU with up to 53% of cases having some association and the other common risk factors are diabetes (21% - 42%) [2,14-20], human immunodeficiency virus infection (1.6% – 22%), chronic kidney or liver disease, obesity, previous spinal surgery [13,14,15,16,18]. The bacterium most frequently responsible for SEA is the S. aureus and particularly, Methicillin-resistant Staphylococcus aureus (MRSA), followed by Methicillin-sensitive Staphylococcus aureus (MSSA), in 43 % and 23% of cases respectively [18,20-22]. The most common mechanism of SEA is hematogenous dissemination (50%), followed by extension from contiguous infected tissues through the vertebral venous plexus (30%) and direct inoculation through the surgical procedure [2,6,23-27]. On the other hand, primary or spontaneous SEA has no evident infective source or history of trauma, surgery, or percutaneous treatment that may have violated the integrity of the spinal epidural space. This SEA is even rarer accounting for 20% of all SEAs [28-30]. The clinical presentation of SEA is specific, characterized by a triad of pyrexia, neck or back pain, and neurological deficit [15]. However, only 8% – 15% of patients have this triad [31-36]. Delayed diagnosis is in about 90% of cases [2,10,33]. The gold standard study to evaluate patients with suspected SEA is an MRI of the entire spine with contrast. The typical findings on MRI include a high signal on T2-Weighted Imaging (T2WI) and a low signal on T1-Weighted Imaging (T1WI) [3]. The enhancement can be homogenous or peripheral. The peripheral or “ring” enhancement may indicate the presence of purulent fluid, the so-called “true abscess”, that is less amenable to antibiotic therapy; that is because the center of the abscess does not receive adequate vascular supply [3]. Broad differential diagnoses for SEAs include vertebral metastasis, epidural hematoma, disc disease (extruded or migrated discs), and vertebral osteomyelitis or osteodiscitis, among others. If initial imaging is nondiagnostic, and the index of suspicion for SEAs remains high, repeating radiological imaging is mandatory. There is no consensus on the optimal timing of repeating imaging studies [2]. Surgical decompression with near-to-total drainage of fluid collection, microbiological analysis, and adjuvant-targeted antibiotic therapy is the gold standard of combined treatment, in order to guarantee the best neurological recovery [18,37-40]. Nevertheless, recent systematic reviews and meta-analyses have shown that, in selected cases, only empirical, neoadjuvant antibiotic therapy can provide superimposable clinical results [41,42]. These contradictory results suggest that further research and a more detailed classification of the various subtypes of SEA are needed to clarify the outcome predictors, the indications, and the surgical timing for SEA. Postoperative recovery depends on age, health status, comorbidities, and history duration; above all, it is crucial to the patient’s neurological status immediately before surgery. In the literature, there is only an article about SEA in patients with COVID-19 [8], dealing with the treatment of six patients with COVID-19 with primary SEA, during the first three months of the SARS-COV2 pandemic. We highlight the main different features of the SEA in COVID-19 patients.
Epidemiological and clinical evidence in COVID-19 patients’ SEA
In the only article about SEA in COVID-19 patients [8,43], the authors treated six patients with primary SEA. Five of them complained of a severe form of SARS-CoV-2 infection; in one patient, the diagnosis was only based on clinical symptoms with a highly suggestive lung CT ground glass opacity appearance; in one case, the SEA developed once the infection was already resolved with high antibody titer; the last two patients presented an almost asymptomatic viral infection, revealed by the serologic tests [8]. These patients presented different characteristics from traditional SEA in no COVID-19 patients (Table 1). In their series, Talamonti, et al. [8] described a younger average age with a similar sex distribution (male-to-female ratio 2:1). The main difference was the fact that none of them was a drug abuser. Three were obese, two were diabetic and only one presented a moderate chronic kidney disease. Another important difference was that nobody developed any form of spondylodiscitis. MSSA was the only responsible pathogen. In three patients MSSA was also subsequently isolated in blood cultures, without clinical evidence of pyogenic infection in other body districts nor presenting signs of sepsis. Interestingly, in literature, MRSA is more frequently involved [44-46]. In this series, only one patient presented the typical onset triad with simultaneous pyrexia, neck or back pain, and neurological deficit. All patients underwent blood tests including blood culture and renal function before the MRI study. Moreover, antibiotics therapy was started according to the infectious disease evaluation. All cases were scheduled for early surgery with the aim of spinal decompression, abscess drainage, and germ isolation; we recorded one case of diffuse deep venous thrombosis with pulmonary embolism in one obese patient. The authors did not register any post-operative neurological recovery. In our experience, we did not observe a direct correlation between the occurrence of SEA and the clinical severity of COVID-19, as evidenced by the lack of a relationship between SEA and mortality. Moreover, when SEA occurred, only three patients were still fighting against active SARS-CoV-2 infection. All these patients had lymphopenia with a mild increase in white blood cell count, specifically neutrophilia; furthermore, five people had previously received immunomodulators or corticosteroids (dexamethasone) to counter the viral infection.
To date is well known that coronavirus is responsible for diffuse endothelial damage [47,48]. The authors wondered if the viral infection could have played a role in damaging the vascular endothelium, thus favoring the vascular penetration of MSSA even in the absence of a clear upper respiratory tract MSSA infection. In this way, MSSA could have reached the correspondent spinal epidural space causing progressive cellulitis of the epidural fat with the ultimate formation of the SEA. Mild immunodeficiency cannot be excluded even in the two patients who were asymptomatic for COVID-19: multiple recent clinical trials in vitro suggest that SARS-CoV-2 causes functional exhaustion of CD8 T-cells and natural killer lymphocytes due to persistent stimulation from the virus, thus inducing T-cell exhaustion. For patients with bacteremia and COVID-19, CD4, and CD8 T-cell functional exhaustion may be the reason why the MSSA can be found in the epidural space and develops a localized abscess, such as in our patients [49].
Radiological evidence in COVID-19 patients’ SEA
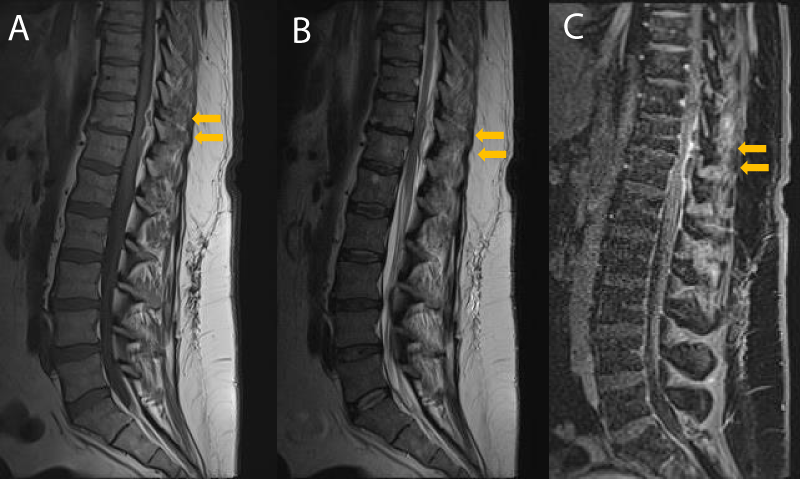
Several differences in radiological findings were highlighted and early identification of these features is mandatory to exclude differential diagnosis and begin early treatment (Table 2). In this series, the cervicothoracic spine was the most involved site. The reason is the large fatty epidural space with a highly represented epidural venous plexus, extensively connected with both epidural lymphatics and vertebral bone veins. In our opinion, there is a significant role in developing abscesses through arterial bacteriaemia and, in this region, thanks to the redundancy of metameric radicular-medullary arteries, the vascular supply is really represented. In literature, two types of enhancement patterns are described: homogenous enhancement, which may correspond to an abscess with an inflammatory tissue in the absence of a purulent collection; and a peripheral or ring enhancement, which may indicate the presence of purulent fluid or “true abscess” [3,50,51]. The ring-enhancing abscesses are less amenable to antibiotic therapy, as the center of the abscess does not receive adequate vascular supply to treat the infection with systemic antibiotics [3,52,53]. COVID-19 SEA is generally active abscesses, with a large cystic component, characterized by a strong hyperintense signal on T2-weighted images with corresponding hypo intensity in T1-weighted images, suggesting a fluid content. (Figure 3). The cystic core is generally enclosed by a soft, fibrous capsule, characterized by high contrast enhancement after gadolinium administration, creating the pattern of enhancing ring. The authors observed that MRI with intravenous administration of gadolinium (Gd) is more sensitive for the diagnosis of a COVID-19-related SEA. Gadolinium-enhanced MR images can aid in the definition of the age and consistency of the abscess: liquid pus is associated with an area of low signal intensity on T1-weighted images, whereas a rim of tissue that enhances after the injection of Gd represents granulation tissue [3]. COVID-19-related SEA was hyperintense relative to the surrounding tissues on diffusion-weighted MRI images and they appeared dark on apparent diffusion coefficient maps. Finally, SEA in COVID-19 patients seems to be not related to spondylodiscitis: no signs of the disc or vertebral body infectious process were observed. Moreover, no indirect signs of spondylodiscitis, such as posterior paraspinal muscle edema, psoas edema, or intervertebral disk signal abnormality, have been reported in these rare entities.
Conclusion
During the COVID-19 pandemic, a new entity of SEA was described. A high incidence of primary SEA was noticed in non-drug abuser COVID-19 patients. Since the outcome of SEA often remains poor, mainly because of delayed diagnosis and treatment, physicians should be aware that COVID-19 patients may have some greater risk of SEA than the general population. Performing an MRI with contrast is mandatory in patients with back pain and new-onset neurological symptoms. A careful study of MRI features is critical for the choice of treatment. Due to current poor evidence about SEA in COVID-19 patients, further studies are needed to validate these preliminary radiological findings.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
- Artenstein AW, Friderici J, Holers A, Lewis D, Fitzgerald J, Visintainer P. Spinal Epidural Abscess in Adults: A 10-Year Clinical Experience at a Tertiary Care Academic Medical Center. Open Forum Infect Dis. 2016 Sep 14;3(4):ofw191. doi: 10.1093/ofid/ofw191. PMID: 28018923; PMCID: PMC5172511.
- Bond A, Manian FA. Spinal Epidural Abscess: A Review with Special Emphasis on Earlier Diagnosis. Biomed Res Int. 2016;2016:1614328. doi: 10.1155/2016/1614328. Epub 2016 Dec 1. PMID: 28044125; PMCID: PMC5156786.
- Thurnher MM. Spinal infections. In: Van Goethem J, van den Hauwe L, Parizel PM, editors. Spinal imaging: diagnostic imaging of the spine and spinal cord. Berlin: Springer Berlin Heidelberg; 2007. p. 521-41.
- Kapeller P, Fazekas F, Krametter D, Koch M, Roob G, Schmidt R, Offenbacher H. Pyogenic infectious spondylitis: clinical, laboratory and MRI features. Eur Neurol. 1997;38(2):94-8. doi: 10.1159/000113167. PMID: 9286631.
- Khan SH, Hussain MS, Griebel RW, Hattingh S. Title comparison of primary and secondary spinal epidural abscesses: a retrospective analysis of 29 cases. Surg Neurol. 2003 Jan;59(1):28-33; discussion 33. doi: 10.1016/s0090-3019(02)00925-4. PMID: 12633952.
- Darouiche RO. Spinal epidural abscess. N Engl J Med. 2006; [355:2012-20. PMID:17093252DOI:10.1056/NEJMra055111]
- Du JY, Schell AJ, Kim CY, Trivedi NN, Ahn UM, Ahn NU. 30-day Mortality Following Surgery for Spinal Epidural Abscess: Incidence, Risk Factors, Predictive Algorithm, and Associated Complications. Spine (Phila Pa 1976). 2019 Apr 15;44(8):E500-E509. doi: 10.1097/BRS.0000000000002875. PMID: 30234819.
- Talamonti G, Colistra D, Crisà F, Cenzato M, Giorgi P, D'Aliberti G. Spinal epidural abscess in COVID-19 patients. J Neurol. 2021 Jul;268(7):2320-2326. doi: 10.1007/s00415-020-10211-z. Epub 2020 Sep 10. PMID: 32910251; PMCID: PMC7482053.
- Farber SH, Murphy KR, Suryadevara CM, Babu R, Yang S, Feng L, Xie J, Perfect JR, Lad SP. Comparing outcomes of early, late, and non-surgical management of intraspinal abscess. J Clin Neurosci. 2017 Feb;36:64-71. doi: 10.1016/j.jocn.2016.10.035. Epub 2016 Nov 9. PMID: 27836393.
- Tuchman A, Pham M, Hsieh PC. The indications and timing for operative management of spinal epidural abscess: literature review and treatment algorithm. Neurosurg Focus. 2014 Aug;37(2):E8. doi: 10.3171/2014.6.FOCUS14261. PMID: 25081968.
- Strauss I, Carmi-Oren N, Hassner A, Shapiro M, Giladi M, Lidar Z. Spinal epidural abscess: in search of reasons for an increased incidence. Isr Med Assoc J. 2013 Sep;15(9):493-6. PMID: 24340840.
- Soehle M, Wallenfang T. Spinal epidural abscesses: clinical manifestations, prognostic factors, and outcomes. Neurosurgery. 2002 Jul;51(1):79-85; discussion 86-7. doi: 10.1097/00006123-200207000-00013. PMID: 12182438.
- Brown PCM, Phillipi GM, King C, Tanski M, Sullivan P. Evaluating new paralysis, mortality, and readmission among subgroups of patients with spinal epidural abscess: A latent class analysis. PLoS One. 2020 Sep 11;15(9):e0238853. doi: 10.1371/journal.pone.0238853. PMID: 32915861; PMCID: PMC7485888.
- Tompkins M, Panuncialman I, Lucas P, Palumbo M. Spinal epidural abscess. J Emerg Med. 2010 Sep;39(3):384-90. doi: 10.1016/j.jemermed.2009.11.001. Epub 2010 Jan 8. PMID: 20060254.
- Reihsaus E, Waldbaur H, Seeling W. Spinal epidural abscess: a meta-analysis of 915 patients. Neurosurg Rev. 2000 Dec;23(4):175-204; discussion 205. doi: 10.1007/pl00011954. PMID: 11153548.
- DeSanto J, Ross JS. Spine infection/inflammation. Radiol Clin North Am. 2011 Jan;49(1):105-27. doi: 10.1016/j.rcl.2010.07.018. PMID: 21111132.
- Hlavin ML, Kaminski HJ, Ross JS, Ganz E. Spinal epidural abscess: a ten-year perspective. Neurosurgery. 1990 Aug;27(2):177-84. PMID: 2385333.
- Karikari IO, Powers CJ, Reynolds RM, Mehta AI, Isaacs RE. Management of a spontaneous spinal epidural abscess: a single-center 10-year experience. Neurosurgery. 2009 Nov;65(5):919-23; discussion 923-4. doi: 10.1227/01.NEU.0000356972.97356.C5. PMID: 19834405.
- Baralo B, Kulkarni M, Ellangovan R, Selko R, Kulkarni A, Guha Roy S, Gilbert M. Cervical, Thoracic, and Lumbar Spine Epidural Abscess: Case Report and Literature Review. Case Rep Infect Dis. 2020 Oct 7;2020:8834589. doi: 10.1155/2020/8834589. PMID: 33101744; PMCID: PMC7568137.
- Ropper AE, Ropper AH. Acute Spinal Cord Compression. N Engl J Med. 2017 Apr 6;376(14):1358-1369. doi: 10.1056/NEJMra1516539. PMID: 28379788.
- Vakili M, Crum-Cianflone NF. Spinal Epidural Abscess: A Series of 101 Cases. Am J Med. 2017 Dec;130(12):1458-1463. doi: 10.1016/j.amjmed.2017.07.017. Epub 2017 Aug 7. PMID: 28797646.
- Arko L 4th, Quach E, Nguyen V, Chang D, Sukul V, Kim BS. Medical and surgical management of spinal epidural abscess: a systematic review. Neurosurg Focus. 2014 Aug;37(2):E4. doi: 10.3171/2014.6.FOCUS14127. PMID: 25081964.
- Chan JJ, Oh JJ. A rare case of multiple spinal epidural abscesses and cauda equina syndrome presenting to the emergency department following acupuncture. Int J Emerg Med. 2016 Dec;9(1):22. doi: 10.1186/s12245-016-0116-5. Epub 2016 Jul 26. PMID: 27456667; PMCID: PMC4960080.
- Godhania V. Lumbar spine osteomyelitis and epidu- ral abscess formation secondary to acupuncture. J Surg Case Rep. 2016; 2016:rjw035. [PMID: 26976275 PMCID: PMC4789537 DOI: 10.1093/jscr/rjw035]
- Lee JH, Cho JH, Jo DJ. Cervical epidural abscess after cupping and acupuncture. Complement Ther Med. 2012 Aug;20(4):228-31. doi: 10.1016/j.ctim.2012.02.009. Epub 2012 Mar 28. PMID: 22579435.
- Reynolds F. Neurological infections after neuraxial anesthesia. Anesthesiol Clin. 2008 Mar;26(1):23-52, v. doi: 10.1016/j.anclin.2007.11.006. PMID: 18319178.
- Okano K, Kondo H, Tsuchiya R, Naruke T, Sato M, Yokoyama R. Spinal epidural abscess associated with epidural catheterization: report of a case and a review of the literature. Jpn J Clin Oncol. 1999 Jan;29(1):49-52. doi: 10.1093/jjco/29.1.49. PMID: 10073152.
- Khan SH, Hussain MS, Griebel RW, Hattingh S. Title comparison of primary and secondary spinal epidural abscesses: a retrospective analysis of 29 cases. Surg Neurol. 2003 Jan;59(1):28-33; discussion 33. doi: 10.1016/s0090-3019(02)00925-4. PMID: 12633952.
- Wang Z, Lenehan B, Itshayek E, Boyd M, Dvorak M, Fisher C, Kwon B, Paquette S, Street J. Primary pyogenic infection of the spine in intravenous drug users: a prospective observational study. Spine (Phila Pa 1976). 2012 Apr 15;37(8):685-92. doi: 10.1097/BRS.0b013e31823b01b8. PMID: 22037525.
- Kitov B, Kehayov I, Davarski A, Stoyanova R. Outcome of Surgical Treatment of Spontaneous Spinal Epidural Abscesses for a 10-year Period. Folia Med (Plovdiv). 2020 Sep 30;62(3):482-489. doi: 10.3897/folmed.62.e49902. PMID: 33009757.
- Moatz B, Michael K, Rhee JM. Spinal epidural abscesses: diagnosis and current treatment options. Semin Spine Surg. 2016; 28:143-9 [doi: 10.31616/asj.2019.0369 PMCID: PMC7595828PMID: 32718133]
- Darouiche RO. Spinal epidural abscess. N Engl J Med. 2006 Nov 9;355(19):2012-20. doi: 10.1056/NEJMra055111. PMID: 17093252.
- Davis DP, Wold RM, Patel RJ, Tran AJ, Tokhi RN, Chan TC, Vilke GM. The clinical presentation and impact of diagnostic delays on emergency department patients with spinal epidural abscess. J Emerg Med. 2004 Apr;26(3):285-91. doi: 10.1016/j.jemermed.2003.11.013. PMID: 15028325.
- Hlavin ML, Kaminski HJ, Ross JS, Ganz E. Spinal epidural abscess: a ten-year perspective. Neurosurgery. 1990 Aug;27(2):177-84. PMID: 2385333.
- Moatz B, Michael K, Rhee JM. Spinal epidural abscesses: diagnosis and current treatment options. Semin Spine Surg. 2016; 28:143-9 [doi: 10.31616/asj.2019.0369 PMCID: PMC7595828PMID: 32718133]
- Tahir MZ, Hassan RU, Enam SA. Management of an extensive spinal epidural abscess from C-1 to the sacrum. Case report. J Neurosurg Spine. 2010 Dec;13(6):780-3. doi: 10.3171/2010.5.SPINE09545. PMID: 21121757.
- Patel AR, Alton TB, Bransford RJ, Lee MJ, Bellabarba CB, Chapman JR. Spinal epidural abscesses: risk factors, medical versus surgical management, a retrospective review of 128 cases. Spine J. 2014 Feb 1;14(2):326-30. doi: 10.1016/j.spinee.2013.10.046. Epub 2013 Nov 12. PMID: 24231778.
- Suppiah S, Meng Y, Fehlings MG, Massicotte EM, Yee A, Shamji MF. How Best to Manage the Spinal Epidural Abscess? A Current Systematic Review. World Neurosurg. 2016 Sep;93:20-8. doi: 10.1016/j.wneu.2016.05.074. Epub 2016 Jun 1. PMID: 27262655.
- Wheeler D, Keiser P, Rigamonti D, Keay S. Medical management of spinal epidural abscesses: case report and review. Clin Infect Dis. 1992 Jul;15(1):22-7. doi: 10.1093/clinids/15.1.22. PMID: 1617070.
- Alton TB, Patel AR, Bransford RJ, Bellabarba C, Lee MJ, Chapman JR. Is there a difference in neurologic outcome in medical versus early operative management of cervical epidural abscesses? Spine J. 2015 Jan 1;15(1):10-7. doi: 10.1016/j.spinee.2014.06.010. Epub 2014 Jun 14. PMID: 24937797.
- Siddiq F, Chowfin A, Tight R, Sahmoun AE, Smego RA Jr. Medical vs surgical management of spinal epidural abscess. Arch Intern Med. 2004 Dec 13-27;164(22):2409-12. doi: 10.1001/archinte.164.22.2409. PMID: 15596629.
- Shweikeh F, Saeed K, Bukavina L, Zyck S, Drazin D, Steinmetz MP. An institutional series and contemporary review of bacterial spinal epidural abscess: current status and future directions. Neurosurg Focus. 2014 Aug;37(2):E9. doi: 10.3171/2014.6.FOCUS14146. PMID: 25081969.
- Sampogna G, Tessitore N, Bianconi T, Leo A, Zarbo M, Montanari E, Spinelli M. Spinal cord dysfunction after COVID-19 infection. Spinal Cord Ser Cases. 2020 Sep 30;6(1):92. doi: 10.1038/s41394-020-00341-x. PMID: 32999271; PMCID: PMC7525226.
- Huang PY, Chen SF, Chang WN, Lu CH, Chuang YC, Tsai NW, Chang CC, Wang HC, Chien CC, Chen SH, Huang CR. Spinal epidural abscess in adults caused by Staphylococcus aureus: clinical characteristics and prognostic factors. Clin Neurol Neurosurg. 2012 Jul;114(6):572-6. doi: 10.1016/j.clineuro.2011.12.006. Epub 2011 Dec 27. PMID: 22206858.
- Shweikeh F, Hussain M, Sangtani A, Issa H, Bashir A, Johnson JP, Markarian GZ. Cervical spine epidural abscess: a single center analytical comparison to the literature. Spinal Cord Ser Cases. 2017 Jul 6;3:17036. doi: 10.1038/scsandc.2017.36. Erratum in: Spinal Cord Ser Cases. 2017 Oct 05;3:17061. PMID: 28690871; PMCID: PMC5498827.
- Ma H, Kim I. Clinical outcomes of spinal epidural abscess. Korean J Spine. 2012 Mar;9(1):6-11. doi: 10.14245/kjs.2012.9.1.6. Epub 2012 Mar 31. PMID: 25983781; PMCID: PMC4432386.
- Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, Kucher N, Studt JD, Sacco C, Bertuzzi A, Sandri MT, Barco S; Humanitas COVID-19 Task Force. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020 Jul;191:9-14. doi: 10.1016/j.thromres.2020.04.024. Epub 2020 Apr 23. PMID: 32353746; PMCID: PMC7177070.
- Carsana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, Zerbi P, Rech R, Colombo R, Antinori S, Corbellino M, Galli M, Catena E, Tosoni A, Gianatti A, Nebuloni M. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. 2020 Oct;20(10):1135-1140. doi: 10.1016/S1473-3099(20)30434-5. Epub 2020 Jun 8. PMID: 32526193; PMCID: PMC7279758.
- Choudhury I, Han H, Manthani K, Gandhi S, Dabhi R. COVID-19 as a Possible Cause of Functional Exhaustion of CD4 and CD8 T-cells and Persistent Cause of Methicillin-Sensitive Staphylococcus aureus Bacteremia. Cureus. 2020 Jul 4;12(7):e9000. doi: 10.7759/cureus.9000. PMID: 32775080; PMCID: PMC7402531.
- Ziu M, Dengler B, Cordell D, Bartanusz V. Diagnosis and management of primary pyogenic spinal infections in intravenous recreational drug users. Neurosurg Focus. 2014 Aug;37(2):E3. doi: 10.3171/2014.6.FOCUS14148. PMID: 25081963.
- Pradilla G, Nagahama Y, Spivak AM, Bydon A, Rigamonti D. Spinal epidural abscess: current diagnosis and management. Curr Infect Dis Rep. 2010 Nov;12(6):484-91. doi: 10.1007/s11908-010-0140-1. PMID: 21308559.
- Sendi P, Bregenzer T, Zimmerli W. Spinal epidural abscess in clinical practice. QJM. 2008 Jan;101(1):1-12. doi: 10.1093/qjmed/hcm100. Epub 2007 Nov 3. PMID: 17982180.
- Parkinson JF, Sekhon LH. Spinal epidural abscess: appearance on magnetic resonance imaging as a guide to surgical management. Report of five cases. Neurosurg Focus. 2004 Dec 15;17(6):E12. doi: 10.3171/foc.2004.17.6.12. PMID: 15636569.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
