Journal of Neurology, Neurological Science and Disorders
Neuroplasticity and neuronal communications in the healthy and in the disease brain
María Pilar González1*, Adrián Macho-González1,2, Alba Garcimartin1,2, María Elvira López-Oliva3, Juana Benedi1 and José Joaquín Merino1
2Departamento de Nutrición y Ciencia de la Alimentación, Spain
3Sección Departamental de Fisiología, Spain
Cite this as
González MP, González AM, Garcimartin A, López-Oliva ME, Benedi J, at al. (2019) Neuroplasticity and neuronal communications in the healthy and in the disease brain. J Neurol Neurol Sci Disord 5(1): 038-046. DOI: 10.17352/jnnsd.000032In this review we explain: 1) the molecular mechanism by which de nervous system establishes communication between all their cells (neurons and glia), 2) the way by which this system organizes its configuration (plasticity); in order to send signals, which form part of our behaviour, memory, thoughts, movements and all the functions that allow us to communicated with our environment and 3) the altered neurotransmission involved in brain diseases or disabilities.
Introduction
The neurotransmission may be defined as the set of biochemical and physiochemical signals, which establishes neuronal communication. Each neuron is connected with numerous other neurons in a very specific shape forming a neuronal network, which may change during the life according to environmental signals. This process is called neuronal plasticity. These changes may be produced in adult and young brains, although during the developing brain is higher as compared to the adult brain [1-3].
The variety of physicochemical signals that neurons may process could lead to a great quantity of transduction mechanisms. However, the evolution has only used a few mechanisms to process all signals. Synaptic plasticity is crucial for regulating synaptic transmission or neuronal connectivity in order to share essential information among neurons and glia cells and maintaining homeostasis in the body.
Neuroplasticity is the capacity of the central nervous system to undergo structural changes as adaptive response against different stimulus. Structural plasticity is the brain ability to change its structure as consequence of learning and learning or structural remodelling under CNS damage in pathological conditions by neuroprotective drugs [4-7].
The individual synaptic connections are constantly removed or ¨remodelled¨ depending on the neurons from its environment. The neuroplasticity is highly regulated by signals from other cells in the nervous system as astrocytes, which maintains the neuronal function and position. This remodelling ability is achieved by genetic, molecular and cellular mechanisms that influence the synaptic connexions and neuronal circuits in the brain under homeostatic and pathological conditions.
The circuitry of the human brain is composed of a trillion neurons and a quadrillion synapses, whose connectivity underlies all human functions as perception, emotion, thought and behaviour. Brain plasticity is not always good. Sometimes generates abnormal brain connexion under brain disease. There are two types of neuronal plasticity: functional and structural. Several mechanisms contribute to brain plasticity including an over-production of neurons and synapses during the early development and apoptosis [8].
This review focuses on synaptic phenomena involved in homeostatic plasticity. We also explain the relevance of neuronal plasticity and the relationship between neurotransmission and physiological dysfunctions. In addition, we discuss a putative modulation of the signalling mechanisms involved in homeostatic functions and neuronal remodelling as well as therapeutic approach against neurological and neurodegenerative disorders.
Synaptic plasticity and function
The neurons are connected among them forming structures named synapses that make them suitable the neuronal intercommunication and govern all vital and cognitive functions.
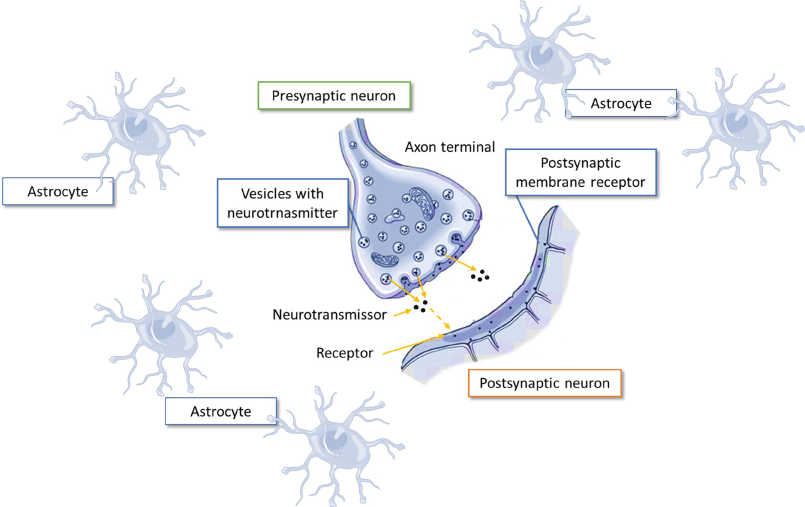
The synapse is a structure formed by the axon terminal of a neuron named presynaptic and another postsynaptic neuron, which are separated by the synaptic clefts. In the axon of the presynaptic neuron neurotransmitters are inside of synaptic vesicles. The postsynaptic neuron is the cell that recruits the neurotransmitter receptors and received their signals. Astrocytes are surrounding the synapses attending several functions, such as the elimination of the neurotransmitter to stop the neurotransmission [7,9-13] or maintaining the position of all cells that make up the cells of the CNS among other (Figure 1).
A single neuron can participate in over 100.000 synapses in the mouse and over one million synapses in the human brain [9]. Astrocytes regulate several aspects in the synapses as formation, maturation and functions taking part in the uptake of some neurotransmitter [14]. In addition, they may control the ionic balance at the synapses including Ca2+ and K+ ions [15,16]. Astrocytes also secrete factors inductors of synaptogenesis and of specific excitatory and inhibitory circuit formation [17-20].
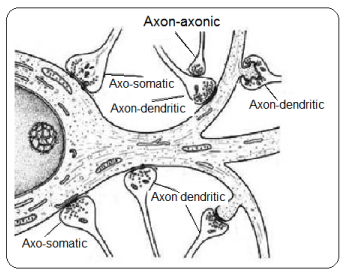
Several synapses types exist, depending on the connection among neurons. These may be a) axo-somatic, when the axon of the presynaptic neuron makes synapsis with the cellular body of the postsynaptic neuron, b) axo-axonic, synapsis between axons, c) axon-dendrite, connection between axons and dendrites (Figure 2).
These communications make possible neuronal transmission and it permits wide possibility or signals among neurons. If this transmission fails, physiological disturbances as lack of movement, coordination and neurological disorders such as dementia, schizophrenia, depression etc. may take place.
A single neuron receives thousands of synaptic inputs. Each neuron communicates with 1000 neurons and may receive, simultaneously, ten times of connections from other neurons. It is estimated that the adult brain has, at least, 1014 synaptic connections.
The synapse is the structure where the neurons establish their communication. Therefore, it is highly regulated not only respect to the neurotransmitter release but also with regard its conformation (synaptic plasticity).
The synapse plasticity includes different facets at different levels: 1) presynaptic levels, 2) neurotransmitter removal and 3) postsynaptic levels.
Presynaptic level
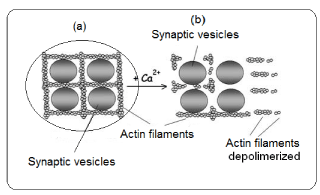
At the presynaptic level, there are sequences of events which conclude with the neurotransmitter release. In the end of nervous terminal, the synaptic vesicle (SV) are docking by proteins from cytoskeleton, mainly actin (Figure 3).
The Ca2+, which has gone inside the neuron through voltage dependent Ca2+ channels, is bound to some cytoskeleton proteins (actin). These actin proteins are polymerized forming filaments which cross each other docking several cellular organelles, between them the SV. In presence of Ca2+, the actin filaments are depolymerized and as consequence the SV remains free and may approach to the presynaptic membrane. In this case, the Ca2+ acts as an intracellular messenger. The sequence of actin depolymerization is as follows. Synapsin, a protein located in the membrane of the SV, is one of the responsible of binding between SV and actin filaments. This protein is implicated in the phosphorylation-dephosphorylation of actin. When Ca+ is elevated, the calmodulin (a protein-kinase) is activated. The active calmodulin phosphorylates the synapsin, being this the inductor of actin depolymerization; Thus, SV is free and ready to migrate toward the presynaptic membrane. The action of Ca, calmodulin and synapsin action on neurotransmission has been studied [21].
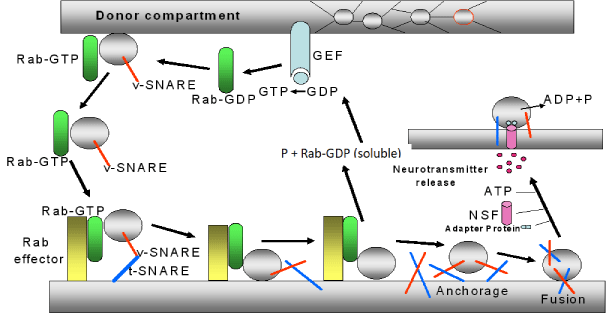
This process of SV approach is highly regulated by proteins from the SV membrane and membrane proteins at the presynaptic neuron. Many of these proteins bind Ca2+; among these, vesicle-associated membrane proteins (VAMPs), synapsin, synaptophisin, synaptotagmin, SNARE (Soluble NSF Attachment Protein Receptor), between then, t- SNARE (target-SNARE) and v-SNARE (vesicular SNARE), etc. The sequence of events involved in the neurotransmitter release is as follow (figure 4):
1) Rab (protein of the GTP-ase family and the Ras subfamily) is a soluble and inactive protein located in the cytoplasm of the nervous terminal (donor compartment of SV). When this protein bounds GTP forms a complex, the Rab-GTP, which it is able to bind to the synaptic vesicle. The formation of GTP is mediated by a guanine nucleotide exchange factor (GEF) located at the presynaptic membrane.
2) The complex Rab-GTP bounds to the proteins vesicular SNARE (v-SNARE,) located in the SV, forming a complex Rab-GTP-SV. This makes that the SV moves toward the presynaptic membrane and recognizes a receptor denominated effecter Rab.
3) Rab-GTP-SV is bounded to this effector (forming Rab-GTP-effector Rab-SV) and also to a group of proteins located in SV and in the cellular membrane (the v-SNARE, located in SV, and the t-SNARE, located in the cellular membrane). This makes that both membranes (the cellular and the vesicular) stay very close.
4) When the SV is near to the presynaptic membrane, both proteins t-SNARE and v-SNARE roll up themselves forming a stable complex denominated trans-SNARE. This causes a greater approximation between SV and presynaptic membrane. After that, the GTP, linked to Rab, is hydrolyzed producing Rab-GDP (soluble), and P. This makes that SV to separate from the Rab-GTP-effector Rab-SV complex to free the SV, which is anchored to the presynaptic membrane.
5) Once this process take place, the SNARE separates from this complex, and a signalling protein, denominated N-ethylmaleimide-sensitive factor (NSF), is activated by ATP with the mediation of an ATP-ase enzyme. NSF is linked to an adapter protein and to SV. In this process, ATP is hydrolyzed and its energy is used to unroll the SNARE (v-SNARE and t-SNARE). Both SNARES and other not well identified proteins (although it could be possible that some of them were synaptophysin and synaptobrevin) give place to a pore formation by which SV may release the neurotransmitter [22-26] (Figure 4).
Elimination of neurotransmitter from the synaptic clefts
The presence of the neurotransmitter in the synaptic space should be very short and the neurotransmitter needs to disappear from this area. This process may be performed by several mechanisms: (a) the released neurotransmitter is removed by enzymes located at the pre and postsynaptic neuron membrane (i.e: acetylcholine) and (b) by neurotransmitter transporters located in neurons and astrocytes situated in the synaptic space (Figure 1). This way is used for amino acid neurotransmitters.
Postsynaptic levels
The neurotransmitter is bound to specific postsynaptic receptors, which respond with specific cellular signals, like the entry of Na+ and Ca2+ ions into the postsynaptic neuron. In this area is where the long-term potentiation (LTP) and the long-term depression (LTD) takes place. The physical and biological mechanism of LTP is not well understood, but several models have been developed. LTP may mean memory consolidation and LTD long-term memory depression. Several forms of LTP exist depending on the brain area and the age of the organism. For example, the molecular mechanism of LTP of the adult hippocampus differs from the young hippocampus [27]. The signals pathways, used by each cell, contributes to the LTP specificity. Like this, some types of hippocampal LTP depend on the NMDA receptor and other depends on the metabotropic glutamate receptor (mGluR) or upon other molecule.
The induction of the LTP and LTD is dependent on the postsynaptic increase in intracellular Ca2+. Ca2+ activates several enzymes as phosphatase, calcineurin, calcium/calmodulin dependent protein kinase II (CaMKII), and protein kinase C (PKC), some of them are necessaries to fire LTP. The activation of these enzymes plays a regulatory role during synaptic plasticity as well as during the induction of the LTP and learning.
Some authors suggest that the LTP induction needs CaMKII activation in the hippocampus, Calmodulin mediates the activation of this enzyme, which previously has been activated by the Ca2+ increase. However, other authors suggest that is not necessary CaCMII activation to induce LTP. Therefore, the PKC activation seems to be necessary for both LTP and memory maintenance [28,29].
LTP is related with the memory mechanism [30,31]. At the end of the XIX century, the scientific believed that the memory was associated to higher number of neurons number but soon they realised that this was not the explanation. In 1894, Santiago Ramon y Cajal was the first researcher in suggesting that the learning was the result of a strengthening of the existing neurons for a better communication between them. Later, in 1949, Donald Hebb proposed that the neurons may generate new connexions induced by metabolic changes which may potentate their ability of communication. The continuous excitation (LTP) may induce a great number of pre and postsynaptic signals, among them metabolic changes which might affect the efficiency in the presynaptic zone, and/or the ability of increase the excitation in the postsynaptic neuron. These assumptions have been partly resolved with the knowledge of the LTP. The LTP was discovered in the hypothalamus but was further identified in other brain structures [32]. There are scientific that assume the existence of different LTP types or mechanism denominated Hebbians, non-Hebbians and anti-Hebbians [33,34]. In the Hebbian mechanism the pre and postsynaptic neurons are required to induce LTP. The non-Hebbian mechanism does not necessarily involve the depolarization of both pre and postsynaptic neurons. In the anti-Hebbian mechanism depolarization of presynaptic neuron and hyperpolarization of the postsynaptic neuron are necessary.
Glutamate as inductor of LTP in the postsynaptic area
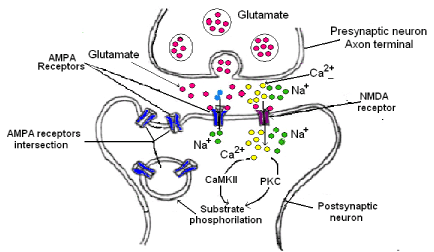
Glutamate is the major excitatory neurotransmitter in the brain. It activates several receptors (ionotropic and metabotropic) at the postsynaptic neurons. The ionotropic receptors NMDA and AMPA are important in the hippocampal synaptic plasticity and LTP induction [35,36]. When NMDA receptor is activated, a great Ca2+ flux takes place inside the postsynaptic neuron. This Ca2+ increase is necessary for LTP induction. However, the NMDA need not only the postsynaptic depolarization for activation but also the presynaptic transmitter release to induce LTP. On the contrary, AMPA receptors are sufficient for the expression of the LTP due to the fact that after its activation, news AMPA receptors could be incorporated to the postsynaptic membrane (Figure 5). The mechanism is as follows:
1. The glutamate release by the presynaptic neuron is bound to NMDA and AMPA receptors in the postsynaptic membrane.
2. AMPA receptor is a Na+ channel; therefore, the Na+ entry induces both membrane depolarization and opening of voltage dependent Ca2+ channels.
3. NMDAR is a Ca2+ channel, then, Ca2+ enters inside the postsynaptic neuron and activates the calmodulin (CaM), this activates calcium/calmodulin dependent protein kinase II (CaMKII) which it is the responsibility that AMPA receptor moves through the membrane postsynaptic leading to more AMPA receptors in this site and as consequence a long time of excitation.
Involvement of glial cells in neuroplasticity: Astrocytes
Astrocytes represent the most abundant population of glia in the mammalian brain and are crucial for the functions of the nervous system as trophic support to neurons [37] and it is also involved in neurotransmission [38]. They establish close contacts with neurons and blood vessel and envelope thousands of synapses [39]. In addition, astrocytes participated actively in the maintenance of the health and activity neuronal [40,41], including regulation of the synaptic formation, transmission, neuromodulation and plasticity. The mechanism by which astrocytes perform these effect is through the release of different molecules like thrombospondin [42], and cholesterol [43] among others.
Astrocytes express several receptors among them the purinergic, adrenergic, glutamatergic and GABAergic [44-47].
Astrocytes remove excess of glutamate after glutamatergic transmission, transforming it in glutamine and returning it to the neurons as glutamate [45]. In addition, astrocytes control the ionic balance at the synapse, including Ca2+ and K+ ions that are crucial for synaptic neurotransmission [44,48].
It has been thought for a long time that astrocytes were not excitable; nevertheless, astrocyte excitability is manifested by the elevation of cytosolic Ca2+ mainly as result of endoplasmic reticulum mobilization of Ca2+ [49]. These results suggest that astrocytes may be capable to mediate in neurotransmission and affect neuronal activity. Astrocytes can release neuroactive substances denominated gliotransmitters that can bind to presynaptic and/or postsynaptic receptors and also can regulate neuronal excitability and synaptic transmission [50-52].These results confirmed than astrocytes are integral elements of the synapse. The gliotransmitter release may be Ca2+ dependent, vesicular release as in neurons, and Ca2+ independent. Astrocytes express synaptotagmin IV and SNARE type proteins which regulated the glutamate and other neurotransmitters release by a vesicular way [53]. No vesicular gliotransitter release is performed through reversion of transporters uptake [54]. It is now accepted that astrocytes and neurons work together, so, the neuronal activity induces gliotransmitters release. This mechanism is performed via G couple receptors. Astrocytes have receptors type couple to G-proteins, therefore, after neurotransmitter release by the presynaptic neuron, this is bounded to its specific receptor (receptor couple to G protein) in the astrocyte, leading to an increase in IP3. IP3 is bounded to IP3 receptor in the endoplasmic reticulum which promote Ca2+ exit from this cellular compartment and Ca2+ lead to rises in the cytosol and as consequence the release mediated by gliotransmitters.
Although Ca2+ dependent signaling pathway in astrocytes are functional in healthy brain tissues, elevation in Ca2+l levels may be associated to neuronal dysfunctions such as epilepsy [55], Alzheimer diseases [56] and stroke [57]. This may signify that the regulation of the gliotransmision is important. Some authors think that this regulation may carry out as consequence of excitotoxic events that are commun to these diseases. These toxics molecules can be release from damage cells [58] and activate microglia and astrocytes, inducing the release of pro-inflammatory cytokines and chemokines [59], that provoke astrocytes damage as consequence of alterations in neuronal functions and death.
Signalling from synapses to nucleus
The feature that gene expression could be mediated via neuronal activity was known through the observation that agonist of the nicotinic acetylcholine receptor and other neurotransmitters that induce membrane depolarization and Ca2+entry through voltage dependent Ca2+ channels also might express c-fos in neurons [60]. Later, different research found that other genes were also lead by this mechanism [61,62]. Among them BDNF regulate survival, dendritic, axonal growth, and excitatory and inhibitory synapses development; Arc controls glutamate receptor endocytosis; Cpg115 regulates survival and dendritic growth among other [63-68]. The main mechanism, inductor of all this, is the increase of intracellular Ca2+ inside the neurons. The intracellular Ca2+ may be caused for several mechanisms like the opening of voltage dependent Ca2+ channels by glutamate through its ionotropic receptors as NMDA and by Ca2+ release from intracellular compartment, the mitochondria and the endoplasmic reticulum. Each one of these mechanics induces different signals, which are the responsible of the expression of different genes. The signals cascades induced by Ca2+ influx include several pathways like Ras/MAPK, the calcium/calmodulin dependent protein kinase, the phosphatase calcineurin and Rac GTPases. These signalling pathways affect the activity of several transcriptional factors involved in the activation of transcription factors that activate the transcription of several hundred of genes which may affect a great variety of biological process.
For instance, L-type voltage dependent Ca2+ channels induces expression of protein kinase A type (AKAP79/150), tyrosine kinase Src and phosphatase calcineurin [69]. Some of these induce phosphorylation of the L-type voltage dependent Ca2+ channels inhibiting the Ca2+ entering through these transports. Calcineurin activation is necessary for the translocation.
NMDA and AMPA receptors have several types of subunits and according that these receptors may be Ca2+ or Na+ channels. This mean that the genes that these receptor express will be different. Calcium influx through NMDA receptor activates receptors such as Rac, GEFs, beta-PIX and Kalirin-7 [70,71]. All this suggests that the signalling mechanism of neurotransmission induces gene expression in the nucleus and when this mechanism falls it may causes several neurological disorders.
Synaptic plasticity may modify gene expression at several levels. High synapses stimulation may send signals to the cellular nucleus to induce RNA synthesis. Synaptic activity also can modify protein synthesis which can act at several important steps during translation.
The neurotransmitters which may be release into synapses are of two types: ionotropic or metabotropic. The binding of an ionotropic neurotransmitter to its receptor induces depolarization and Ca2+ entry, through voltage dependent Ca2+ channels. However, the metabotropic receptor, as it is a Gq coupled receptor, mediated the increase of intracellular Ca2+ via intracellular compartments (endoplasmic reticulum). Each route of Ca2+ influx induces the synthesis of different gene. For instance BDNF is highly induced by Ca2+ entry through L- voltage dependent Ca2+ channels but not by other Ca2+ dependent channels or Ca2+ entry through NMDA receptor in excitatory neurons [72]. Other signals may be transport from the synapses to the cellular nucleus through the slow retrograde axonal transport [73]
Disease caused by dysfunction of the neurotransmission and therapeutic strategies
If all of the above are taken into account it can considered that the neurotransmission failure can affect all the organs of the living being since, through nerve transmission, the “brain” can control all the functions of the organism not only at the physiological level but also at the psychic level. So, nervous transmission dysfunction can lead to disability or dementia and madness. Neurotransmission failure is not only due to alterations of the release mechanisms and/or dysfunctions of neurotransmitter but also may be due to other disorders such as myelin destruction or cellular death induced by several causes.
The most common symptoms due to neurotransmission failures are:
a) Physical such as paralysis and loss of movements that will lead the patients to disabilities, sometime very deep, that can lead to a total dependence.
b) Physical with memory losses, attacks, depressions and madness.
In this review, examples of diseases that occur due to neurotransmission dysfunctions will be presented.
Myasthenia syndromes: The most of diseases from congenital myasthenia syndromes (CNS) are characterized, among other symptoms, by cognitive dysfunctions and astrocytes abnormalities [74], suggesting a crucial role of these cells in normal cognitive and function. CNS pathologies are a group of neurological diseases characterized by weakness and fatigability [75]. Many of these diseases are due to genes encoding proteins of the presynaptic region some of them associated with the SNARE complex [76]
Brain ischemia: During brain ischemia there are a lack of O2 and glucose and as consequence low ATP because neurons containing few storage glycogen, then the neurons dye; however, astrocytes may maintained during more time the production of energy (ATP) under hypoxia conditions because they have higher glycogen as reserve [77]. Therefore, during ischemia astrocytes may increase the neurons survival at expense of the lactate submitted by these cells as result of anaerobic glucose degradation [78].
Alzheimer disease: The Alzheimer disease is characterized by the decrease of the cholinergic system then, the use of inhibitors of acetylcholinesterase enzyme was considered as a good treatment to improve cholinergic neuronal function. Astrocytes express nicotinic receptors from the α7 type (α−nAChR) [79], therefore, the use of inhibitor of AChE (AChEIs) make that astrocytes kept during more time the acetylcholine in the synaptic cleft, favoring the cholinergic transmission. In addition, AChEIs also reduce ROS production and apoptosis [80]. Makatani et al, 2017 [81] consider that protective mechanisms due also to nicotinic actions through PI3K-Akt pathways may also be efficient in Alzheimer, although this disease is incurable.
Schizophrenia: Schizophrenia (SZ) is a complex and heterogeneous psychiatric disorder characterized by a group of symptoms including delusions, hallucinations, impaired cognitive functioning, disorganized speech and behaviour [82]. The Schizophrenia etiology is unknown, the illness implies genetic, environmental and epigenetic factors. Several studies have related alterations in the neurotransmission mediated by biogenic amines, and at dysfunctions in glutamic, dopamine and GABAergic neurotransmission [83, 84].
Epilepsy: Epilepsy is a disease known in the times of Hippocrates being call and ‘shaking palsy’. During many times was considered as the result of a demonic possession. In 1929 the invention of electroencephalography transformed the epilepsy into a well-defined syndrome and due to this method it was demonstrated that this disease was due to abnormal brain waves which produced hypersynchronous discharge of large populations of neurons in the brain. Epilepsy may be produced by imbalances in neurotransmitters and mutations in their receptors, ion channels, transporters and altered network connectivity (plasticity) [85, 86]. It is classified into two categories genetic and idiopathic. Genetic may be caused for changes in a single protein while idiopathic is due to a polygenic disorder.
Several biological changes have been described during epileptogenesis including gliosis [87], uncontrolled inflammation [88], disruption of the blood-brain barrier [89], neurodegeneration [90], changes in neuronal circuits [91], aberrant neurogenesis, promotes release of glutamate with the subsequent neuronal cells death [92]. All these alteration are the responsible of the horrifying syndrome in this disease.
Down syndrome: Down syndrome is a genetic disease that occurs due to an aneuploidy of human chromosome 21. This leads to a set of cognitive anomalies and learning deficits. The disease course with deficiencies in GABA-ergic neurotransmission, noradrenergic neuronal loss, anomalous glutamatergic and NMDA receptor signalling, mitochondrial dysfunction and inflammation [93].
Other disease related with neurotransmission are Parkinson, with failure and death of dopaminergic neurons. Autism characterized by a spectrum of disorder characterized by impaired social communication, social interaction and repetitive behaviours [94].
Conclusion
Since brain disorders affects neurotransmission it is crucial to understand signalling pathways by which neurodegeneration alters neurotransmitter levels. Thus, the analysis of neurotransmission could help to understand possible role of neurotransmitters in neuroprotection by pharmacological approaches. In addition, the neurotransmission is important for understanding learning and memory processes and also neurorepair mechanisms in the injured brain. Neurotransmitters like glutamate, GABA, etc could be associated markers of neuroprotective drugs in neurodegenerative diseases.
- Peters A, Palay SL, Webster H (1991) de F. The Fine Structure of the Nervous System: The Cells and Their Processes. New York: Oxford University Press.
- Rustom A, Saffrich R, Markovic I, Walther O, Gerdes HH (2004) Nanotubular highways for intercellular organelle transport. Science 303: 1007-1010. Link: http://bit.ly/2TL69Bv
- Kandel ER, Schwarz JH, Jessell TM (1991) Principles of neural Science. Amsterdam: Elsevier.
- Hodgkin AL (1964) The Conduction of the Nervous Impulse. Spingfield I.L: Charles C Thomas. Link: http://bit.ly/2z6NmHv
- Devlin TM (1999) Membranas Biológicas: Estructura y Transporte a través de membranas. In Devlin T.M. (ed) Bioquímica 1: 179-216.
- Bezanilla F (2000) The voltage sensor in voltage dependent ion channels. Physiol Rev 80: 555-592. Link: http://bit.ly/31SMG4Z
- Jan LY, Jan IN (1997) Voltage-gated and inwardly rectifiying potassium channels. J Physiol Rev 505: 267-282. Link: http://bit.ly/2Z1RJD9
- Johnston MV, Nishimura A, Harum K, Pekar J, Blue ME (2001) Sculpting the developing brain. Adv Pediatr 48: 1-38. Link: http://bit.ly/2Hc0vDq
- Perea G, Araque A (2007) Astrocytes potentiate transmitter release at single hippocampal synapses. Science 317: 1083-1086. Link: http://bit.ly/2Mq0Tmf
- Santello M, Volterra A (2009) Synaptic modulation by astrocytes via Ca2+-dependent glutamate release. Neuroscience 158: 253-259. Link: http://bit.ly/2Z7RjKN
- Petrelli F, Bezzi P (2016) Novel insights into gliotransmitters. Curr Opin Pharmacol 26: 138-145. Link: http://bit.ly/2Z6XTlD
- De Pitta M, Brunel N (2016) Modulation of synaptic plasticity by glutamatergic gliotransmission: a modeling study. Neural Plast 2016: 7607924. Link: http://bit.ly/2ZgrHXP
- Atwood BK, Lovinger DM, Matur BN (2004) Presynaptic long-termdepression mediated by Gi/o-coupled receptors. Trends Neurosci 37: 663-673. Link: http://bit.ly/31TmBCV
- Tani H, Dulla CG, Farzampour Z, Taylor-Weiner A, Huguenard JR, et al. (2014) A Local Glutamate-Glutamine Cycle Sustains Synaptic Excitatory Transmitter Release. Neuron 81: 888-900. Link: http://bit.ly/2TJgHkD
- Djukic B, Casper KB, Philpot BD, Chin LS, McCarthy KD (2007) Conditional Knock-Out of Kir4.1 Leads to Glial Membrane Depolarization, Inhibition of Potassium and Glutamate Uptake, and Enhanced Short-Term Synaptic Potentiation. J Neurosci 27: 1354 -1365. Link: http://bit.ly/2KTZy3N
- Sibille J, Pannasch U, Rouach N (2014) Astroglial potassium clearance contributes to short-term plasticity of synaptically evoked currents at the tripartite synapse. J Physiol 592: 87-102. Link: http://bit.ly/2Z9q1Ud
- Buosi AS, Matias I, Araujo APB, Batista C, Gomes FCA (2017) Heterogeneity in Synaptogenic Profile of Astrocytes from Different Brain Regions. Mol Neurobiol 55: 751-762. Link: http://bit.ly/30rMYPG
- Mayorquin LC, Rodriguez AV, Sutachaan JJ, Albarracín SL (2018) Connexin-Mediated Functional and Metabolic Coupling Between Astrocytes and Neurons. Font Mol Neurosoci 11: 1-10. Link: http://bit.ly/30ir2GI
- Diniz LP, Almeida JC, Tortelli V, Vargas Lopes C, Setti-Perdigão P, et al. (2012) Astrocyte-induced synaptogenesis is mediated by transforming growth factor beta signaling through modulation of D-serine levels in cerebral cortex neurons. J Biol Chem 287: 41432-41445. Link: http://bit.ly/31KjVas
- Diniz LP, Tortelli V, Garcia MN, Stipursky J, Kahn SA, et al, (2014) Astrocyte transforming growth factor beta 1 promotes inhibitory synapse formation via CaM kinase II signaling. Glia 62: 1917-1931. Link: http://bit.ly/2KJU64V
- Llinás R, Gruner JA, Sugimori M, McGuinness TL, Greengard P (1991) Regulation by synapsin I and Ca(2+)-calmodulin-dependent . J Physiol 436: 257-282. Link: http://bit.ly/30rMNUw
- Pavlos NJ, Reinhard J (2011) Distinct yet overlapping roles of Rab GTPases on synaptic vesicles. Small GTPases 2: 77-81. Link: http://bit.ly/2P3TkUu
- Schlager MA, Hoogenraad CC (2009) Basic mechanisms for recognition and transport of synaptic cargos. Molecular Brain 2: 1-12. Link: http://bit.ly/31OVQiN
- Binotti B, Jahn R, En Chua JJ (2016) Functions of Rab Proteins at Presynaptic Sites. Cells 6: 1-10. Link: http://bit.ly/2Hc05Nm
- Stahll B, Chou JH, Li C, Sudhof TC, Jahn R (1996) Rab3 reversibly recruits rabphilin to synaptic vesicles by a mechanism analogous to raf recruitment by ras. The EMBO Journal 15: 1799-1809. Link: http://bit.ly/31PuFUS
- Jae-Bong PARK, Jun-Sub KIM, Jae-Yong LEE, Jaebong KIM, Ji-Yeon SEO, et al. (2002). GTP binds to Rab3A in a complex with Ca2+/calmodulin. Biochem J 362: 651-657. Link: http://bit.ly/2Zgp20F
- Yasuda H, Barth AL, Stellwagen D, Malenka RC (2003) A developmental switch in the signaling cascades for LTP induction". Nat Neurosci 6: 15-16. Link: http://bit.ly/2TIvHiM
- Malenka RC, Kauer JA, Perkel DJ, Mauk MD, Kelly PT, et al. (1989) An essential role for postsynaptic calmodulin and protein kinase activity in long-term potentiation. Nature 340: 554-557. Link: http://bit.ly/2zabXeo
- Malinow R, Schulman H, Tsien RW (1989) Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science 245: 862-866. Link: http://bit.ly/2Hea0C2
- Shema R, Sacktor TC, Dudai Y (2007) Rapid erasure of long-term memory associations in the cortex by an inhibitor of PKM zeta. Science 317: 951-953. Link: http://bit.ly/2HbGRYw
- Pignatelli M, Roy DS. Tonegawa S, Ryan TJ (2015) Memory engram storage and retrieval. Current Opinion in Neurobiology 35: 101-109. Link: http://bit.ly/2KRY2z7
- Wang DJ, Su LD, Wang YN, Yang D, Sun ChL, et al (2014) Long-Term Potentiation at Cerebellar Parallel Fiber–Purkinje Cell Synapses Requires Presynaptic and Postsynaptic Signaling Cascades. The Journal of Neuroscience 34: 2355-2364. Link: http://bit.ly/2P2KVAq
- Yasuda H, Barth AL, Stellwagen D, Malenka RC (2003) A developmental switch in the signaling cascades for LTP induction. Nat Neurosci 6: 15-16. Link: http://bit.ly/2TIvHiM
- Sacktor TC (2011) How does PKMζ maintain long-term memory? Nat Rev Neurosci 12: 9-15. Link: http://bit.ly/2KFpZeM
- Kessey K, Mogul DJ (1997) NMDA-Independent LTP by adenosine A2 receptor-mediated postsynaptic AMPA potentiation in hypocampus. J Neurophysiol 78: 1965-1972. Link: http://bit.ly/33CYCcN
- Kullmann DM, Erdemli G, Asztély F (1996) LTP of AMPA and NMDA Receptor–Mediated Signals: Evidence for Presynaptic Expression and Extrasynaptic Glutamate Spill-Over. Neuron 17: 461-474. Link: http://bit.ly/2KU9WZu
- Bank G (1980) Trophic interactions between astroglial cells and hippocampal neurons in culture. Science 209: 809-810. Link: http://bit.ly/2MoR1t0
- Murphy-Royal C, Dupuis J, Groc L, Oliet SHR (2017) Astroglial glutamate transporters in the brain: Regulating neurotransmitter homeostasis and synaptic transmission. J Neurosci Res 95: 2440-2151. Link: http://bit.ly/2HaG35R
- Genoud C, Quairiaux C, Steiner P, Hirling H, Welker EC, et al. (2006) Plasticity of astrocytic coverage and glutamate transporter expression in adult mouse cortex. PLoS Biol 4: 2057-2064. Link: http://bit.ly/30gRFMr
- Sofroniew MV, Vinters HV (2010) Astrocytes: Biology and pathology. Acta Neuropathol 119: 7-35. Link: http://bit.ly/2Z1Jkzo
- Allaman I, Bélanger M, Magistretti PJ (2001) Astrocyte-neuron metabolic relationships: For better and for worse. Trends Neurosci 34: 76-87. Link: http://bit.ly/2ZdaO0f
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, et al. (2005) Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120: 421-433. Link: http://bit.ly/2ZdaNtd
- Mauch DH, Nagler K, Schumacher S, Göritz C, Müller EC, et al. (2001) CNS synaptogenesis promoted by gliaderived cholesterol. Science 294: 1354-1357. Link: http://bit.ly/2KMOXaI
- Djukic B, Casper KB, Philpot BD, Hin LS, McCarthy KD (2007) Conditional Knock-Out of Kir4.1 Leads to Glial Membrane Depolarization, Inhibition of Potassium and Glutamate Uptake, and Enhanced Short-Term Synaptic Potentiation. J Neurosc 27: 11354-11365. Link: http://bit.ly/2KTZy3N
- Tani H, Dulla CG, Farzampour Z, Taylor-Weiner A, Huguenard JR, et al (2014) Local Glutamate-Glutamine Cycle Sustains Synaptic Excitatory Transmitter Release. Neuron 81: 888-900. Link: http://bit.ly/2TJgHkD
- Duffy S, MacVicar BA (1996) Adrenergic calcium signaling in astrocyte networks within the hippocampal slice. J Neurosci 15: 5535-5550. Link: http://bit.ly/30hwERF
- Porter JT, McCarthy KD (1997) Astrocytic neurotransmitter receptors in situ and in vivo. Prog Neurobiol 51: 439-455. Link: http://bit.ly/2YZxK7X
- Sibille J, Pannasch U, Rouach N (2014) Astroglial potassium clearance contributes to short-term plasticity of synaptically evoked currents at the tripartite synapse. J Physiol 592: 87-102. Link: http://bit.ly/2Z9q1Ud
- Dani JV, Chernjavsky A, Smith SJ (1992) Neuronal activity triggers calcium waves in hippocampal astrocyte networks. Neuron 8: 429-440. Link: http://bit.ly/2HeMipD
- Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, et al. (2014) Gliotransmitters travel in time and space. Neuron 81: 728-739. Link: http://bit.ly/2Nglnxg
- Eroglu N, Barres BA (2010) Regulation of synaptic connectivity by glia. Nature 468: 223-231. Link: http://bit.ly/2P64Mic
- Araque, AG, Carmignoto PG, Haydon PG, Oliet SH, Robitaille R, et al. (2014) Gliotransmitters travel in time and space. Neuron 81: 728-739. Link: http://bit.ly/2Nglnxg
- Hamilton NB, Attwell D (2010) Do astrocytes really exocytose neurotransmitter? Nature Reviews Neuroscience 11: 227-238. Link: http://bit.ly/2Z0R5pw
- Schousboe A, Sarup A, Bak LK, Waagepetersen SH, Larsson OM (2004) Role of astrocytic transport processes in glutamatergic and GABAergic neurotransmission. Neurochem Int 45: 521-527. Link: http://bit.ly/31PKlYt
- Ding S, Fellin T, Zhu Y, Lee SY, Auberson YP, et al. (2007) Enhanced astrocytic Ca2C signals contribute to neuronal excitotoxicity after status epilepticus. J Neurosci 27: 10674-10684. Link: http://bit.ly/2NfT5Di
- Kuchibhotla KV, Lattarulo CR, Hyman BT, Backai BJ (2009) Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science 323: 1211-1215. Link: http://bit.ly/2Mm6Bp7
- Rakers C, Schmid M, Petzold GC (2017) TRPV4 channels contribute to calcium transients in astrocytes and neurons during peri-infarct depolarizations in a stroke model. Glia 65: 1550-1561. Link: http://bit.ly/2z8ZVSI
- Takeuchi H, Suzumura A (2014) Gap junctions and hemichannels composed of connexins: potential therapeutic targets for neurodegenerative diseases. Front Cell Neurosci 8: 189. Link: http://bit.ly/33CX0zL
- Orellana JA, Sáez PJ, Shoji KF, Schalper KA, Palacios.Prado N, et al. (2009) Modulation of brain hemichannels and gap junction channels by pro-inflammatory agents and their possible role in neurodegeneration. Antioxid Redox Signal 11: 369-399. Link: http://bit.ly/2KRvExf
- Greenberg ME, Ziff EB Greene LA (1986) Stimulation of neuronal acetylcholine receptors induces rapid gene transcription. Science 234: 80-83. Link: http://bit.ly/2TIQ1jY
- Altar CA, Laeng P, Jurata LW, Brockman JA, Lemire A, et al. (2004) Electroconvulsive seizures regulate gene expression of distinct neurotrophic signaling pathways. J Neurosci 24: 2667-2677. Link: http://bit.ly/2NfGEr5
- Li H, Gu X, Dawson VL, Dawson TM (2004) Identification of calcium- and nitric oxide-regulated genes by differential analysis of library expression (DAzLE). Proc Natl Acad Sci USA 101: 647-652. Link: http://bit.ly/2Zf84zw
- Cantallops I, Haas K, Cline HT (2000) Postsynaptic CPG15 promotes synaptic maturation and presynaptic axon arbor elaboration in vivo. Nat Neurosci 3: 1004-1011. Link: http://bit.ly/2KFohKn
- Chowdhury S, Shepherd JD, Okuno O, Lyford G, Petralia RS, et al (2006) Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron 52: 445-459. Link: http://bit.ly/2ZgmOyl
- Kang H, Schuman EM (1995) Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science 267: 1658-1662. Link: http://bit.ly/2ZeBgqg
- Korte M, Carroll P, Wolf E, Brem G, Thoenem H, et al (1995) Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci USA 92: 8856-8860. Link: http://bit.ly/2z63AjZ
- McAllister AK, Katz LC, Lo DC (1997) Opposing roles for endogenous BDNF and NT-3 in regulating cortical dendritic growth. Neuron 18: 767-778. Link: http://bit.ly/2MspyGz
- Rial Verde EM, Lee-Osbourne J, Worley PF, Malinow R, Cline HT (2006) Increased expression of the immediate-early gene arc/arg3.1 reduces AMPA receptor-mediated synaptic transmission. Neuron 52: 461-474. Link: http://bit.ly/2TNSauT
- Oliveria SF, Dell’Acqua ML, Sather WA (2007) AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron 55: 261-275. Link: http://bit.ly/2KFha4s
- Xie Z, Srivastava DP, Photowala H, Kai, L, Cahill ME, et al. (2007) Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron 56: 640-656. Link: http://bit.ly/2HcK8qv
- Saneyoshi T, Wayman G, Fortin D, Davare M, Hpshi N, et al. (2008) Activity-dependent synaptogenesis: regulation by a CaM-kinasae kinase/CaM-Kinase I/betaPIX signaling complex. Neuron 57: 94-107. Link: http://bit.ly/2HbXJhu
- Ghosh A, Carnahan J, Greenberg ME (1994) Requirement for BDNF in activity-dependent survival of cortical neurons. Science 263: 1618-1623. Link: http://bit.ly/2YWraiJ
- Ch’ng TH, Martin KC (2011) Synapse-to-nucleus signaling. Curr Opin Neurobiol 21: 345-352. Link: http://bit.ly/31LJZSD
- Engel AG, Shen XM, Selcen D, Sine SM (2015) Congenital myasthenic syndromes: Pathogenesis, diagnosis, and treatment. Lancet Neurol 4: 420-434. Link: http://bit.ly/2z8YMKU
- Shen XM, Scola RH, Lorenzoni PJ, Kay CS, Werneeck LC, et al. (2017) Novel synaptobrevin-1 mutation causes fatal congenital myasthenic syndrome. Ann Clin Transl Neurol 4: 130-138. Link: http://bit.ly/2HdvUWq
- Waites CL, Garner CC (2011) Presynaptic function in health and disease. Trend in Neurosci 34: 326-337. Link: http://bit.ly/2TJevtp
- Panickar KS, Norenberg MD (2005) Astrocytes in cerebral ischemic injury: Morphological and general considerations. Glia 50: 287-298. Link: http://bit.ly/2KRV39T
- Narayanan SV, Perez-Pinzon MA (2017) Ischemic preconditioning treatment of astrocytes transfers ischemic tolerance to neurons. Cond Med 1: 2-8. Link: http://bit.ly/30h5QRv
- Teaktong T, Graham A, Court J, Perry R, Jaros E, et al. (2003) Alzheimer's disease is associated with a selective increase in alpha7 nicotinic acetylcholine receptor immunoreactivity in astrocytes. Glia 41: 207-211. Link: http://bit.ly/2z6YzI3
- Liu Y, Zeng X, Hui Y, Zhu C, Wu J, et al. (2015) Activation of alpha7 nicotinic acetylcholine receptors protects astrocytes against oxidative stress‐induced apoptosis: Implications for Parkinson's disease. Neuropharmacology 91: 87-96. Link: http://bit.ly/2z9zV9U
- Makitani K, Nakagawa S, Izumi Y, Akaike A, Kume T (2017) Inhibitory effect of donepezil on bradykinin-induced increase in the intracellular calcium concentration in cultured cortical astrocytes. J Pharmacol Sci 134: 37-44. Link: http://bit.ly/2MpAuVr
- Patel KR, Cherian J, Gohil K, Atkinson D (2014) Schizophrenia: overview and treatment options. Pharm Ther 39: 638-645. Link: http://bit.ly/2P3LtGj
- Howes O, McCutcheon R, Stone J (2015) Glutamate and dopamine in schizophrenia: an update for the 21st century. J Psychopharmacol 29: 97-115. Link: http://bit.ly/31JmsBC
- Lieberman Ja, Kinon BJ, Loebel AD (1990) Dopaminergic mechanisms in idiopathic and drug-induced psychoses. Schizophr Bull 16: 97-110. Link: http://bit.ly/2KJRfZL
- Patel DC, Tewari BP, Chaunsali L, Sontheimer H (2019) Neuron–glia interactions in the pathophysiology of epilepsy. Nature Reviews Neuroscience 20: 282-297. Link: https://go.nature.com/2HcJHfR
- Kaplan DI, Isom LL, Petrou S (2016) Role of sodium channels in epilepsy. Cold Spring Harb Perspect Med 6: 11-17. Link: http://bit.ly/2Mnu6OL
- Coulter DA, Steinhauser C (20115) Role of astrocytes in epilepsy. Cold Spring Harb Perspect Med 5: 1-12. Link: http://bit.ly/2NgimgG
- Dingledine R, Varvel NH, Dudek FE (2014) When and how do seizures kill neurons, and is cell death relevant to epileptogenesis? Adv Exp Med Biol 813: 109-122. Link: http://bit.ly/2P4z7xH
- van Vliet EA, da Costa Araújo S, Redeker S, van Schaik R, Aronica E, et al. (2007) Blood- brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain 130: 521-534. Link: http://bit.ly/2YZTWPh
- Jessberger S, Parent JM (2015) Epilepsy and adult neurogenesis. Cold Spring Harb Perspect Biol 7: 1-10. Link: http://bit.ly/2Zf5j5s
- Goldberg EM, Coulter DA (2013) Mechanisms of epileptogenesis: a convergence on neural circuit dysfunction. Nat Rev Neurosci 14: 337-349. Link: http://bit.ly/2NgN356
- Eid T, Williamson A, Lee TS, Petroff OA, de lanerolle NC (2008) Glutamate and astrocytes key players in human mesial temporal lobe epilepsy? Epilepsia 49: 42-52. Link: http://bit.ly/33EUuc5
- Vacca RA, Bawari S, Valenti D, Tewari D, Nabavi SF, et al. (2019) Down syndrome: Neurobiological alterations and therapeutic targets. Neurosci Biobehav Rev 98:234-255. Link: http://bit.ly/30dCwLS
- Guang S, Pang N, Deng X, Yang L, He F (2018) Synaptopathology Involved in Autism Spectrum Disorder. Frontiers in Cellular Neuroscience 12: 1-16. Link: http://bit.ly/2KWmfnW
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
