Annals of Alzheimer's and Dementia Care
Self-prevention and self-management of the behavioral and psychological symptoms of dementia: A case study
Yohko Maki*
Cite this as
Maki Y (2021) Self-prevention and self-management of the behavioral and psychological symptoms of dementia: A case study. Ann Alzheimers Dement Care 5(1): 011-019. DOI: 10.17352/aadc.000019Copyright
© 2021 Maki Y. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Behavioral And Psychological Symptoms of Dementia (BPSD) are common in individuals with dementia and are associated with poor Quality of Life (QOL) and increased care burden for family members. Managing BPSD, particularly violent behaviors, is essential to improve the QOL for persons with dementia and their families. A 10-week intervention was conducted to empower individuals with Mild Cognitive Impairment (MCI) or early dementia to manage their BPSD independently. Two men participated in the intervention with their wives; both were violent toward their wives. Questionnaire surveys were completed before and at the end of the intervention by the participants and their wives. Case 1 with MCI acknowledged his cognitive decline, expressed self-awareness regarding his violence, and elected to stop his violent behavior to live happier with his family members. Both he and his wife reported improved QOL, and his wife reported reduced violence and care burden. Case 2 with Alzheimer’s disease dementia was unmotivated to stop his violent behavior due to declined memory function and lacked awareness of his behavior. While he reported increased QOL throughout the intervention, his wife reported increased violence and care burdens. These findings suggest that when addressing BPSD in persons with dementia, recollection and self-awareness of behaviors is critical for encouraging the objective identification of violent behaviors and increasing motivation to modify behaviors. Therefore, in cases wherein individuals can reflect on their behaviors, encouraging self-management can be effective in preventing BPSD. However, when they are incapable of reflecting on their behaviors, other measures should be taken. In such cases, rather than management, encouragement and strengthening harmonious behaviors may be effective in improving their own and others’ QOL.
Introduction
Aside from cognitive functions, people with dementia experience various Behavioral and Psychological Symptoms of Dementia (BPSD), including irritability, anxiety, apathy, disinhibition, delusions, hallucinations, and sleep or appetite changes [1]. Approximately 97% of persons living with dementia experience at least one BPSD [2,3], and the presence of BPSD is associated with poor outcomes, such as decreased Quality of Life (QOL) for both persons diagnosed with dementia and their caregivers, increasing caregiver burden, long-term hospital stays, institutionalization, and misuse of medications [4,5].
Among the BPSD, aggressive behaviors may present notable challenges for the caregivers of persons with dementia [6] to manage as many of these behaviors begin with relatively calm behavior but then escalated and develop into violence [7]. Thus, identifying the triggers for these behaviors based on assumptions from previous behaviors is reported to be helpful [6,8], as is taking preventative measures for de-escalation. However, in actual cases, identifying triggers is challenging because the manifestation of BPSD is multifaceted with various complicated factors [1]. Thus, antipsychotic medications are often prescribed, in cases when their behaviors represent a significant safety risk for persons with dementia due to its side effects, such as serious extrapyramidal side effects, including Parkinsonian symptoms (e.g., bradykinesia, akinesia, tremor, and muscle rigidity) [9] and increased mortality [10,11]. Thus, there is still a need to devise non-pharmacological approaches to manage BPSD to improve the QOL of persons with dementia and their caregivers [12].
Self-prevention and management of BPSD for improving QOL
While efforts to manage BPSD have focused on finding the causes, persons with dementia tend to be treated as passive care recipients or those whose behaviors are managed. However, it would be desirable for persons with dementia to be as proactive in preventing BPSD as possible to improve their own and their families’ QOL [13].
Awareness of behavioral psychological symptoms of dementia
A prerequisite of behavioral self-management is that persons with dementia have sufficient cognitive function to remember what they did and said and to recognize and modify their behavior, which requires memory and self-awareness. It is well known that memory function deteriorates in persons with Alzheimer’s Disease Dementia (ADD), as does self-awareness.
Anosognosia, the lack of self-awareness, is a common symptom of ADD [14-18]. As the disease progresses, persons with dementia have increased difficulty reflecting on their own actions objectively. Persons with ADD often have increasing difficulties in making themselves understood due to language dysfunction, leading some to resort to violence to force others to meet their demands. If they suffer from memory dysfunction and anosognosia, they easily forget what they did and said. Even if they remember what they did, they may have difficulty evaluating their own behaviors objectively due to anosognosia.
Thus, it is critical to reflect upon their behaviors and relationships with others at the stage of Mild Cognitive Impairment (MCI) or in the early stages of dementia when capacities of memory and self-awareness are preserved. The advantage of early diagnosis is the ability to allow them to reflect on ways to help themselves live better with progressive dementia [19]. This case study reports two cases, both of whom regularly engaged in violent behavior before the onset of MCI—one with an awareness of violence and one without. Case 1 was a man diagnosed with MCI due to ADD who was 74 years old, and Case 2 was a man diagnosed with ADD who was 78 years old (both wives were 74 years old).
Methods
Intervention: Group intervention
The author conducted a group intervention for older adults with MCI or mild ADD and their spouses. The inclusion criteria included having a diagnosis of MCI or ADD, producing and understanding speech normally, and having an assessed Mini-Mental State Examination (MMSE) [20] score above 20 (range 0–30). The exclusion criterion was having a diagnosis of any psychiatric disease other than MCI or ADD. The intervention consisted of 10 weekly group meetings with four pairs. The other participants included a woman with MCI (MMSE score = 26), aged 76 and her husband aged 78, and a man diagnosed with MCI (MMSE score = 30) who was aged 86 and his wife aged 86.
The purpose of the group meeting was to empower participants to reflect on the source of happiness and empower them to realize the importance of relationships with those close to them, including families and friends who supported them, as having a source of happiness and maintaining good relationships can be significant to live well with dementia [13].
In the first group session, participants were expected to reflect on their own source of happiness using the SMILE chart (Self-Management of autonomous Interdependent Life Empowerment; Figure 1), a model for self-management support [13]. The SMILE approach asks persons with dementia and their families, “What makes you happy?” and encourages them—along with others in their life—to take the initiative in improving their emotional and social health. This process emphasizes self-determination and self-management, guiding persons with dementia to independently establish a holistic goal for their own lives (i.e., the answer to, “What makes you happy?”) and to consider how they can accomplish it in terms of eight components. Reorganizing one’s lifestyle to encourage physical exercise and mental stimulation reportedly helps to protect against dementia onset and progression [21]. Social participation has similar effects in epidemiological research [22] and a narrative review [23]. Volunteering reportedly confers similar benefits [24].
From the outset, SMILE aims to instill interdependence over independence and emphasizes the psychological aspects of self-affirmation, reciprocity, and social reward. Its goal is to achieve “autonomous interdependence,” valuing autonomy (i.e., the ability to make one’s own decisions) over independence as people lose independence as dementia progresses. Moreover, SMILE is designed to encourage gratitude—both giving and receiving gratitude—to present participants’ relationships with others as reciprocal, allowing them to feel needed and affirm their own existence as defined by those relationships. People perceive recognition by others as a form of social reward, which boosts their motivation [25].
SMILE is also intended to give participants fresh recognition of their interdependent relationships by having them take inventory of their lives. Someone with progressive dementia will start to encounter obstacles in their life with time. SMILE does not focus on techniques to help resolve such troubles; instead, it emphasizes cultivating trust between the participants and those around them with the implicit message, “whatever might happen going forward, can be overcome by asking for help from those around you (i.e., your social support), instead of trying to solve the issues by yourself.” A person cannot prepare for all the hardships that dementia might bring; however, when a problem does occur, trust can foster a willingness to proactively search for a solution, including asking others for help. The name was partially inspired by Stage 7e of the Functional Assessment Staging Tool (FAST), an instrument used to assess ADD—“loss of ability to smile” [26]. The idea behind the name is that a person with severe ADD nearing the end of life should still retain the ability to smile until the point they lose consciousness. This was considered a worthwhile goal because a person should be able to remain in good social health until their final days despite the inevitable loss of independence associated with dementia when they are supported by their interdependent relationships with others. Then, they were empowered to consider the challenges and obstacles to living better with MCI/dementia and determine the measures to overcome them independently. In addition, the participants were requested to express gratitude to their spouses every day. As secure relationships can be a critical factor to living well with MCI/dementia, they were expected to realize what support they need to live well and maintain co-beneficial relationships with those who support them.
From the second to ninth sessions, participants freely shared how they tried to cope with challenges, how they tried to enrich their daily living during the past week, exchanged feedback and thoughts, and encouraged each other. In the last session, the participants were requested to reflect on how to live with MCI/dementia (Table 1).
Outcomes
Sense of coherence (SOC) was adopted as the primary outcome of the intervention. Both the persons with dementia and their family members completed the Antonovsky’s SOC scale (score range 0–91, with a higher score indicating higher SOC) [27] at the start of (i.e., baseline) and after the 10-week intervention. This salutogenic model regards a person’s health as subject to the influence of three factors: Comprehensibility , the extent to which one can understand their condition and environment, Manageability, the extent to which they believe they can manage things, and Meaningfulness, a perception of deeper significance in one’s daily endeavors. SOC is recommended as an indicator of social health in mild dementia [28] and resilience to manage the burden of care in dementia [29-33].
The other variables were also assessed, including subjective well-being using the World Health Organization–Five Well-Being Index (WHO-5; range 0–25, with a higher score indicating higher well-being) [34], hope using the Hearth Hope Scale (range 0–48, with higher scores indicating higher hope) [35], gratitude using the Gratitude Questionnaire (GQ-6; range 0–35, higher scores indicating higher gratitude) [36], loneliness with the University of California Los Angeles Loneliness Scale (range 0–80; higher scores indicating higher loneliness) [37], and social support using the Multidimensional Scale of Perceived Social Support (range 0–84, higher scores indicating higher social support) [38].
The severity of BPSD was measured using the caregiver’s report using the Neuropsychiatric Inventory Brief Questionnaire Form (NPI-Q; range 0–180, with higher scores indicating higher BPSD) [39], and burden of care was measured using the Zarit Burden Interview (ZBI; range 0–32, higher scores indicating higher care burden) [40]. As violence was not assessed on the NPI-Q, the frequency and severity of violence were measured based on the family’s report.
Ethical consideration
This intervention was approved by the National Center for Geriatrics and Gerontology’s Conflict of Interest and Ethics Committee (Approval No.1154).
Results
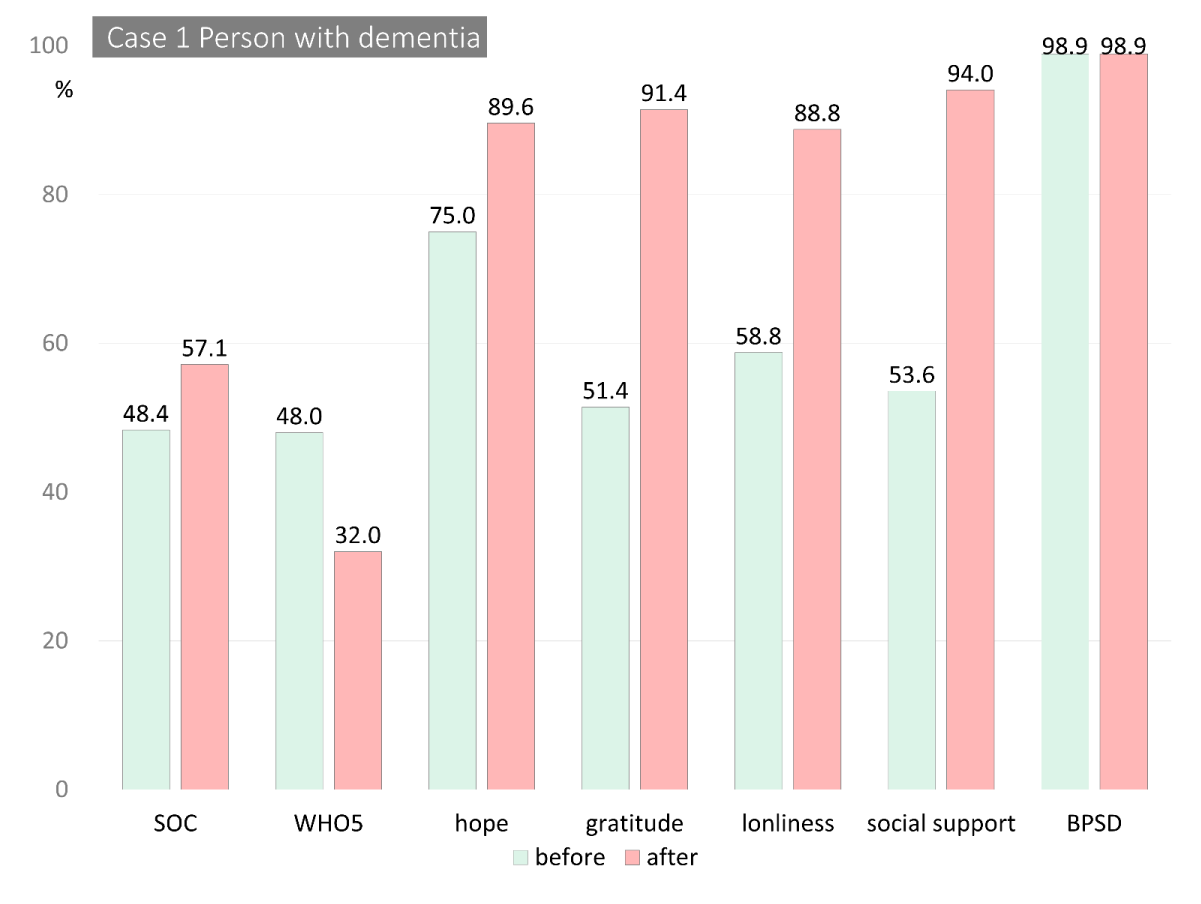
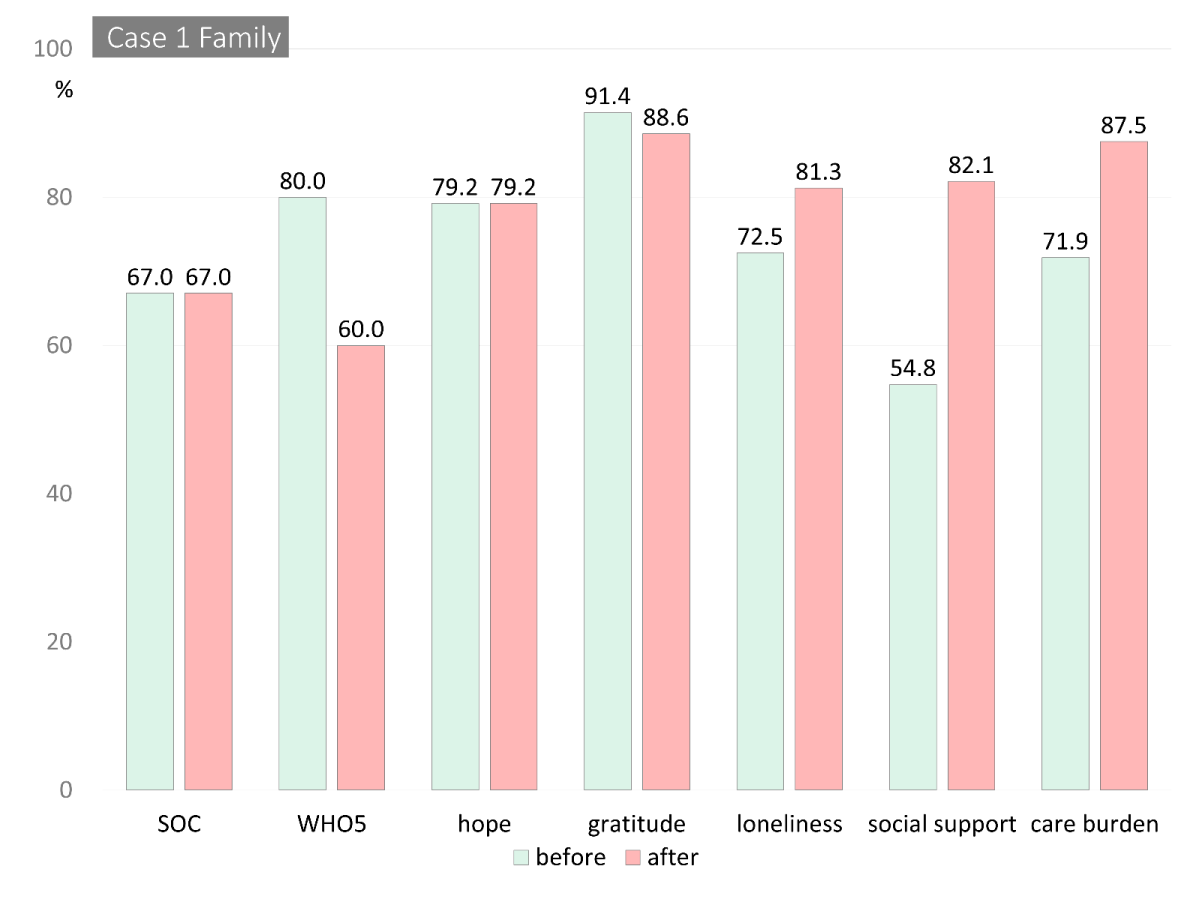
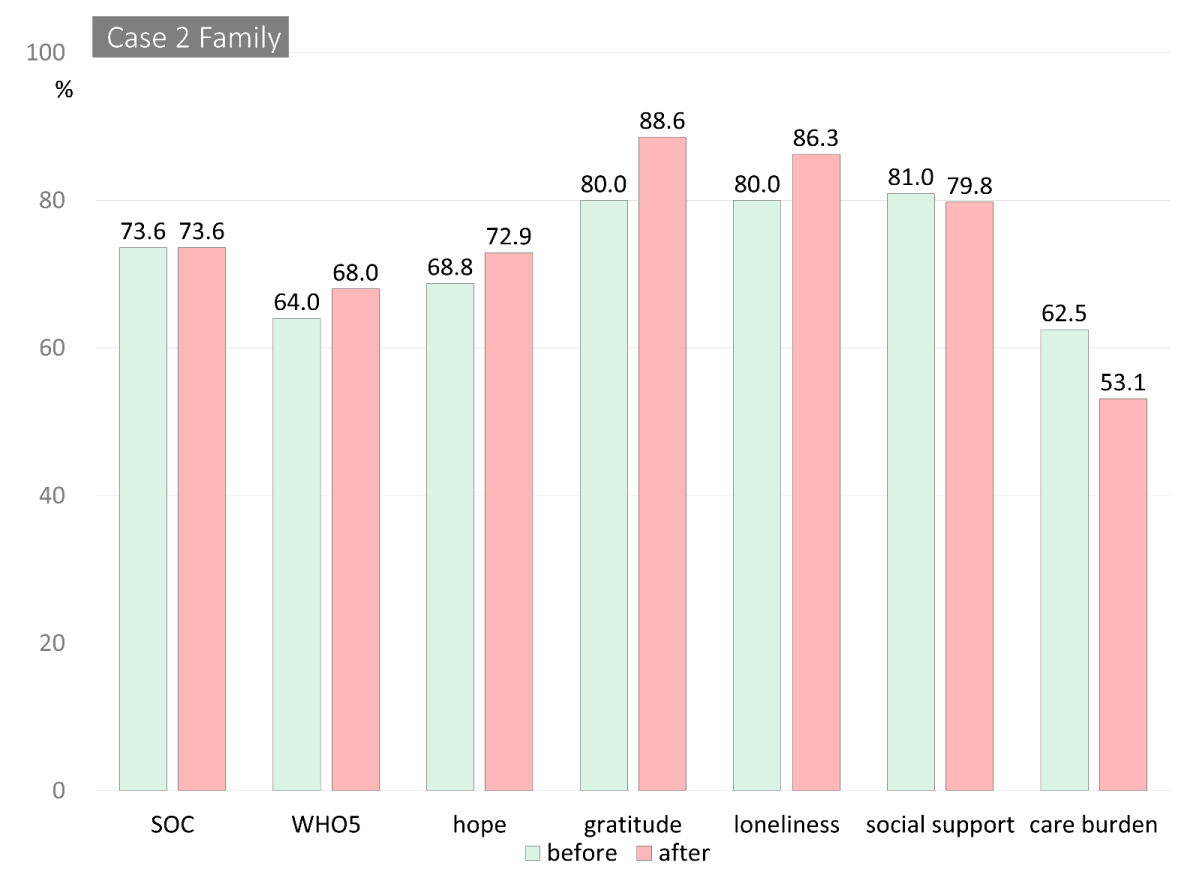
The total scores from the measures for both cases are shown in Table 2. Figure 2 shows changes in the scores from pre- to post-intervention. The scores were reported as percentiles, and the loneliness, BPSD, and care burden scores were reversed to show improvement from pre- to post-intervention.
Case 1
At the beginning of the group meeting, the participant’s violence was reported as being severe, occurring approximately once a week, only toward his wife. Table 1 shows his sources of happiness and responses during the intervention process. His stated sources of happiness were to continue vegetable gardening and engage in volunteer security patrols within the community. However, he shared that he was aware of his declining cognitive function and expressed anxiety regarding his ability to continue engaging in these two identified sources of happiness. He said that he felt some vague maladaptation regarding his ability to communicate with others and would need support in the near future to continue participating in these activities.
In the third session, he suddenly expressed an intention to quit engaging in violent behavior. He said that he needed understanding and cooperation with his wife and that his wife was reluctant to support him due to his violent behavior. He recognized that this violence toward his wife was an obstacle to living better with his wife and decided to stop his violent behavior. He reported that he had wanted to quit being violent numerous times previously but had been unable to do so. However, he realized that due to his declining function, this could be his last chance to modify his behaviors; thus, he needed support from the group members. He also decided to keep a one-line diary to express his gratitude to his wife, although his wife said that she had no feelings of gratitude for him. In every meeting after the fourth meeting, he shared how he attempted to manage his temper and asked for advice and support from the group members; he stated that he continued to write in the diary until his wife received his gratitude. For ten weeks, he succeeded in avoiding violence toward his wife, and his wife began to think of cooperating with him.
Regarding SOC, his Comprehensibility score improved from 15 to 23, while his scores for Manageability and Meaningfulness were almost unchanged (14 to 13, 15 to 16, respectively). His social support (45 to 79), hope (36 to 43), gratitude (18 to 32), and loneliness (33 to 9) scores were improved, but his subjective well-being score decreased (12 to 8). He had insomnia, and the item, “I woke up feeling fresh and rested,” was rated as 0, and the item, “I have felt active and vigorous,” was rated as 1 due to his fatigue.
As for his wife, her reported care burden improved from 9 to 4 points over the course of the intervention. In addition, her self-reported social support (46 to 69), loneliness (22 to 15) scores improved, while her subjective well-being decreased (20 to 15). Her ratings for hope, gratitude, and BPSD, other than violent behavior, were nearly unchanged (Figures 2A & 2B).
Case 2
For Case 2, the severity of violence was augmented, possibly owing to his difficulties controlling the rage. Table 1 shows his source of happiness—he enjoyed a community sports team as his source of happiness. He believed that he was the team leader despite his cognitive impairments and repeatedly shared this, although according to his wife, he was already unable to act in this capacity, and his perception of being the team’s leader might be maladaptive for the team. As he was unaware of his limitations and cognitive impairment, he did not ask for advice or support. He wanted to have a good relationship with his wife despite being unaware of his violent behavior. He was not pretending that he was not violent; rather, he entirely forgot his violent behavior that occurred a few moments previously. Because he was unaware of his violent behavior, he was unmotivated to desist this behavior. His scores on the subjective questionnaire all showed an improvement, although the reported BPSD had worsened (3 to 8; Figures 2C & 2D).
Discussion
Self-prevention and awareness
Case 1’s self-awareness was well preserved; he was aware of his violent tendencies and could learn to control them over the ten-week intervention with encouragement from the group. The intervention’s structure was likely a contributing factor, as it required him to report his process to the group and receive weekly advice for the duration of the intervention. He also explicitly stated each week that he needed his wife’s support and cooperation to discontinue his violent behavior, and she also expressed her experiences and thoughts regarding her husband’s behavior. Regarding SOC, his Comprehensibility score improved from 15 to 23. He clearly recognized his situation and declining cognitive function, could face these difficulties, and asked for support from his wife and the group members every week. After the intervention, his self-reported scores for social support, sense of hope, gratitude, and loneliness improved. His progress over the intervention suggested that he recognized that he could live well with support from his surroundings (i.e., social support), and accordingly, he reported decreased loneliness and increased gratitude to others. Overcoming his violent behavior could have positively led to improved resilience and being able to continue to challenge his declining functioning, which improved his sense of hope for living with MCI. His wife also reported improvements in social support and loneliness and reduced care burden.
In contrast to Case 1, the intervention was found to have no effect on Case 2’s violent outbursts. He experienced memory decline and could not remember what he had done when he saw the bruise on his wife’s arm immediately after being violent. He appeared to suffer from a decline in self-awareness. Due to his declined memory and self-awareness, it was difficult to motivate him to try and control his behavior.
As for the questionnaires, he reported an increase in all the items. A decline in response reliability is common in questionnaire studies, including research with persons with dementia who have anosognosia. Anosognosia appears to suppress the disease’s impact, and those with anosognosia tend to overestimate themselves [41,42]. However, outside informants and the self-perceptions of QOL of persons with dementia have also been found to differ substantially, and to date, neither source of information has been established as being more accurate [43]. Case 2 appeared to experience anosognosia, making it difficult to ascertain that positive changes on questionnaire scores were intervention effects.
Empowering self-management in those with self-awareness
As with Case 1, for those with sufficient self-awareness to engage in self-reflection and be motivated to modify their behaviors to live better, empowering self-decision-making and self-management can effectively modify behaviors. Thus, in the case of violence, they can decide to modify their behaviors and eliminate inappropriate habits, making it possible for them to be empowered by their self-decision-making and self-management.
One way to live better with MCI/dementia may be to identify the source of happiness, which involves confirming the will to live better in the context of declining function and being willing to receive the necessary support from others. It is desirable to consider the challenges to living better (comprehensibility), decide to overcome the challenges and modify behaviors with support from others (manageability), and find meaning in modifying behaviors (meaningfulness) [21]. For a meaningful life with dementia and improved QOL, mutually beneficial relationships with those close to them are critical factors for persons with dementia.
An empowerment approach to strengthen appropriate behaviors for those with insufficient self-awareness
As in Case 2, when self-awareness has declined, it can be difficult to motivate persons with dementia to modify their behaviors as they cannot reflect upon their behavior objectively and may not even remember their behavior. Therefore, empowering self-management cannot be effective in modifying their behaviors based on self-reflection.
Instead of trying to manage behaviors, strengthening their spontaneous behaviors can be effective. After the intervention, he began to use day services for persons with dementia to have opportunities for social interaction. Formal caregivers are typically well-trained to strengthen good communication of persons with dementia. Additionally, because his source of happiness was his participation in a community sports team, Case 2’s wife became involved with the sports team to provide support. Because Case 2 had resorted to violence for a long period of time, and it is difficult to manage habitual violence without internal motivation, stopping habitual violence might require longer time. However, persons with dementia are likely to benefit from being empowered and strengthening their spontaneous words and actions to maintain good relationships with others [44,45].
Conclusions and clinical implications
Social relationships with those close to them are critical for persons with dementia to live well with dementia. The opportunity to independently improve one’s QOL is thought to be a merit of an early diagnosis of dementia [19]. During the early stage of dementia, when self-awareness is retained, empowering self-management is recommended for independently reviewing behaviors and attempting to maintain good relationships with those around them to help those with dementia live better with their diagnosis throughout the course of the disease. Once a person with dementia has difficulties with self-reflection, they may find it difficult to objectively review their behaviors and be motivated to modify these behaviors. At such a stage, persons with dementia may benefit from being empowered and strengthening their communication to be motivated to maintain good communication with others. As dementia affects independence, relationships with others and social support are critical for those with dementia to improve their QOL. Therefore, not limiting interventions to the management of BPSD and focusing on strengthening good communication is desirable to help individuals live better with dementia.
The author is grateful to persons with mild cognitive impairment or dementia and their families who are a source of inspiration.
Funding
This study was supported by Grants-in-Aid for Scientific Research (# 19K11432).
- Cerejeira J, Lagarto L, Mukaetova-Ladinska EB (2012) Behavioral and psychological symptoms of dementia. Front Neurol 3: 73. https://bit.ly/3EmfNBF
- Scales K, Zimmerman S, Miller SJ (2018) Evidence-based nonpharmacological practices to address behavioral and psychological symptoms of dementia. Gerontologist 58: S88–S102. https://bit.ly/3hzoHSI
- Steinberg M, Shao H, Zandi P, Lyketsos CG, Welsh-Bohmer KA, et al. (2008) Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: The Cache County Study. Int J Geriatr Psychiatry 23: 170–177. https://bit.ly/3ClW03z
- Feast A, Moniz-Cook E, Stoner C, Charlesworth G, Orrell M (2016) A systematic review of the relationship between behavioral and psychological symptoms (BPSD) and caregiver well-being. Int Psychogeriatr 28: 1761–1774. https://bit.ly/3zfUnCn
- Feast A, Orrell M, Charlesworth G, Melunsky N, Poland F, et al. (2016) Behavioural and psychological symptoms in dementia and the challenges for family carers: Systematic review. Br J Psychiatry 208: 429-434. https://bit.ly/3CgZ0hE
- Paschali M, Kamp D, Reichmann C, Jaenner M, Lange-Asschenfeldt C, et al. (2018) A systematic evaluation of impulsive-aggressive behavior in psychogeriatric inpatients using the Staff Observation Aggression Scale-Revision (SOAS-R). Int Psychogeriatr 30: 61-68. https://bit.ly/2XkXGev
- Woods DL, Rapp CG, Beck C (2004) Special section— Behavioral symptoms of dementia: their measurement and intervention. Escalation/de-escalation patterns of behavioral symptoms of persons with dementia. Aging & Mental Health 8: 126-132. https://bit.ly/3kghFEc
- Toda D, Tsukasaki K, Itatani T, Kyota K, Hino S, et al. (2018) Predictors of potentially harmful behaviour by family caregivers towards patients treated for behavioural and psychological symptoms of dementia in Japan. Psychogeriatrics 18: 357-364. https://bit.ly/3nBQJk9
- Ohno Y, Kunisawa N, Shimizu S (2019) Antipsychotic treatment of behavioral and psychological symptoms of dementia (BPSD): Management of extrapyramidal side effects. Front Pharmacol 10: 1045. https://bit.ly/3nEiCb9
- Maust DT, Kim HM, Seyfried LS, Chiang C, Kavanagh J, et al. (2015) Antipsychotics, other psychotropics, and the risk of death in patients with dementia: Number needed to harm. JAMA Psychiatry 72: 438–445. https://bit.ly/2XuPusn
- Bessey LJ, Walaszek A (2019) Management of behavioral and psychological symptoms of dementia. Curr Psychiatry Rep 21: 66. https://bit.ly/3nEiwjN
- Akpınar Söylemez B, Küçükgüçlü O, Akyol MA, Işık AT (2020) Quality of life and factors affecting it in patients with Alzheimer’s disease: A cross-sectional study. Health Qual Life Outcomes 18: 304. https://bit.ly/3zcUT3Z
- Maki Y (2019) Proposal for the empowerment of interdependent self-management support for people with dementia. J Geriatr Care Res 6: 3–8. https://bit.ly/398ofGg
- Brandt M, de Carvalho RLS. Belfort T, Dourado MCN (2018) Metamemory monitoring in Alzheimer’s disease: A systematic review. Dementia Neuropsychologia 12: 337-352. https://bit.ly/3Cgy8y5
- Shaked D, Farrell M, Huey E, Metcalfe J, Cines S, et al. (2014) Cognitive correlates of metamemory in Alzheimer’s disease. Neuropsychology 28: 695–705. https://bit.ly/3nDQrte
- Morris RG, Mograbi DC (2013) Anosognosia, autobiographical memory and self knowledge in Alzheimer’s disease. Cortex 49: 1553–1565. https://bit.ly/397PRvg
- Mograbi DC, Brown RG, Morris RG (2009) Anosognosia in Alzheimer’s disease—The petrified self. Conscious Cogn 18: 989–1003. https://bit.ly/2YQP67N
- Souchay C (2007) Metamemory in Alzheimer’s disease. Cortex 43: 987–1003. https://bit.ly/3tXzUBz
- Alzheimer Disease International, Importance of early diagnosis. Link: https://bit.ly/3EmWrMB
- Folstein MF, Folstein SE. McHugh PR (1975) “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189-198. https://bit.ly/3tSwFLC
- Winblad B, Amouyel P, Andrieu S, Ballard C, Brayne C, et al. (2016) Defeating Alzheimer's disease and other dementias: a priority for European science and society. Lancet Neurol 15: 455–532. https://bit.ly/3hCJ2GD
- Mangialasche F, Kivipelto M, Solomon A, Fratiglioni L (2012) Dementia prevention: current epidemiological evidence and future perspective. Alzheimers Res Ther 4: 6. https://bit.ly/3Ci1nkk
- Wajman JR, Mansur LL, Yassuda MS (2018) Lifestyle patterns as a modifiable risk factor for late-life cognitive decline: A narrative review regarding dementia prevention. Curr Aging Sci 11: 90–99. https://bit.ly/3kfD9Rr
- Carlson MC, Kuo JH, Chuang YF, Varma VR, Harris G, et al. (2015) Impact of the Baltimore Experience Corps Trial on cortical and hippocampal volumes. Alzheimer’s Dement 11: 1340–1348. https://bit.ly/3Enaj9O
- World Health Organization. World report on ageing and health. Link: https://bit.ly/3nRg5uH
- Reisberg B (1988) Functional assessment staging (FAST). Psychopharmacol Bull 24: 653–659. https://bit.ly/3kcZC1j
- Antonovsky A (1993) The structure and properties of the sense of coherence scale. Social Science & Medicine 36: 725-733. https://bit.ly/3tHaMic
- Dröes RM, Chattat R, Diaz A, Gove D, Graff M, et al. (2017) Social health and dementia: A European consensus on the operationalization of the concept and directions for research and practice. Aging & Mental Health 21: 4-17. https://bit.ly/3EkOfN5
- Marques MJ, Woods B, Hopper L, Jelley H, Irving K, et al. (2019) Relationship quality and sense of coherence in dementia: Results of a European cohort study. Int J Geriatr Psychiatry 34: 745–755. https://bit.ly/3hBE5Ou
- Matsushita M, Ishikawa T, Koyama A, Hasegawa N, Ichimi N, et al. (2014) Is sense of coherence helpful in coping with caregiver burden for dementia? Psychogeriatrics 14: 87–92. https://bit.ly/3tKEnHk
- Välimäki T, Martikainen J, Hongisto K, Fraunberg M, Hallikainen I, et al. (2014) Decreasing sense of coherence and its determinants in spousal caregivers of persons with mild Alzheimer’s disease in three year follow-up: ALSOVA study. Int Psychogeriatr 26: 1211–1220. https://bit.ly/3AhC9BZ
- Orgeta V, Sterzo EL (2013) Sense of coherence, burden, and affective symptoms in family carers of people with dementia. Int Psychogeriatr 25: 973–980. https://bit.ly/3CdCdTR
- Andrén S, Elmståhl S (2008) The relationship between caregiver burden, caregivers’ perceived health and their sense of coherence in caring for elders with dementia. J Clin Nurs 17: 790–799. https://bit.ly/3Cmz6sV
- Topp CW, Ostergaard SD, Sondergaard S, Bech P (2015) The WHO-5 Well-Being Index: A systematic review of the literature. Psychother Psychosom 84: 167–176. https://bit.ly/2XugFnc
- Vandecreek L, Nye C, Herth K (1994) Where there’s life, There’s hope, and where there is hope, there is. J Relig Health 33: 51–59. https://bit.ly/3tIuB8E
- McCullough ME, Emmons RA, Tsang J (2002) The grateful disposition: A conceptual and empirical topography. J Pers Soc Psychol 82: 112–127. https://bit.ly/39a7mLc
- Russell D, Peplau LA, Ferguson ML (1978) Developing a measure of loneliness. J Pers Assess 42: 290-294. https://bit.ly/3nH5DFN
- Dahlem NW, Zimet GD, Walker RR (1991) The Multidimensional Scale of Perceived Social Support: A confirmation study. J Clin Psychol 47: 756–761. https://bit.ly/39eA7GR
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, et al. (1994) The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 44: 2308–2314. https://bit.ly/3Agr3Nz
- Zarit SH, Reever KE, Bach-Peterson J (1980) Relatives of the impaired elderly: Correlates of feelings of burden. Gerontologist 20: 649–655. https://bit.ly/3ElBm5i
- dos Santos SB, Rocha GP, Fernandez LL, de Padua AC, Reppold CT (2018) Association of lower spiritual well-being, social support, self-esteem, subjective well-being, optimism and hope scores with mild cognitive impairment and mild dementia. Front Psychol 9: 371. https://bit.ly/3nBMogQ
- Verhülsdonk S, Quack R, Höft B, Lange-Asschenfeldt C, Supprian T (2013) Anosognosia and depression in patients with Alzheimer’s dementia. Arch Gerontol Geriatr 57: 282–287. https://bit.ly/3EoVl2Y
- Ready RE, Ott BR, Grace J (2004) Patient versus informant perspectives of quality of life in mild cognitive impairment and Alzheimer’s disease. Int J Geriatr Psychiatry 19: 256–265. https://bit.ly/2VL7opT
- Maki Y, Sakurai T, Okochi J, Yamaguchi H, Toba K (2018) Rehabilitation to live better with dementia. Geriatr Gerontol Int 18: 1529–1536. https://bit.ly/3AgqZgN
- Maki Y (2021) Ikigai interventions for primary, secondary, and tertiary prevention of dementia. Aging and Health Research 1: 100026. Link: https://bit.ly/3AhS431

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
