Annals of Alzheimer's and Dementia Care
Roles of demographics, anthropometric and metabolic syndrome on cognition among mid adults from rural population in Nigeria
Valentine A Ucheagwu1*, Cyriacus Ajaelu2, Paul C Okoli3, Jesse Ossai2, Philip C Ofojebe2 and Rita Ugokwe-Joseph2
2Department of Psychology, Nnamdi Azikiwe University, Awka, Nigeria
3Department of Internal Medicine, Enugu State University of Technology, College of Medicine, Nigeria
Cite this as
Ucheagwu VA, Ajaelu C, Okoli PC, Ossai J, Ofojebe PC, et al. (2019) Roles of demographics, anthropometric and metabolic syndrome on cognition among mid adults from rural population in Nigeria. Ann Alzheimers Dement Care 3(1): 003-010. DOI: 10.17352/aadc.000007There is increased interest in cognitive aging among experts in brain sciences and gerontology as a result of improvement in health sciences leading to longeveity. However, there are very limited studies on this area in Nigeria and some parts of Africa. Our study was to contribute to this area of science with African population, hence we investigated the roles of demographics, anthropometry and metabolic syndrome (Mets) on cognition in mid adults from rural part of Nigeria. Our participants were 172 middle aged adults from a rural community in Anambra state Nigeria. Their age range were between 50 – 70 years with a mean age of 57.48 and SD age of 5.48. The Montreal Cognitive Assessment (MoCA) Symbol Digit Modality Test (SDMT) and Digit Symbol Test (DST) were used to assess the participants’ cognition. The anthropometric measures assessed were the body mass index (BMI), height, weight and waist circumference while the Mets assessed were blood sugar level and total cholesterol and blood pressure. Gender, occupation and education were included as demographic factors assessed in the study. The results show the BMI, weight and waist circumference as predictors of general cognition, but the blood pressure and height were not significant predictors. Equally, mixed findings were got on processing speed. The variables measured were not significant predictors of SDMT as a measure of processing speed while they to significant extent predicted processing speed when DST was used.
Introduction
Cognitive health among mid and late adult population is very important for their overall wellbeing and adjustment. Interest on how to promote healthy cognition as people age has been major concern to health care. Metabolic syndrome (mets) is a cluster of biochemical and physiological conditions associated with the development of cardiovascular disease [1], and type 2 diabetes. Among the biochemical conditions may include abdominal obesity, high blood pressure, high serum triglyceride and low high density lipoprotein (HDL) levels.
In a selected review, Yates, Sweat and Convit [2], describe the literature on the impact of mets on brain and cognition in adults and adolescents. In their review, most studies found associations between mets and cognitive dysfunction with adults having multiple cognitive domains affected while adolescents were mostly in executive functioning. Yates’ et al., [2], review implicated mets in ischemic stroke, white matter alterations and altered brain metabolism. For adolescents, mets factors were linked to volume losses in the hippocampus and frontal lobes. Overall, Yates and Colleagues review show that mets negatively impacts cognitive performance and brain structure. Its potential explanatory models include impaired vascular reactivity and abnormal brain lipid metabolism [2]. Yaffe, Kanaya, Lindquist, Simonsick, Harris, Shorr, Tylavsky and Newman [3], equally showed that elders with metabolic syndrome were more likely to have cognitive impairment compared with those without metabolic syndrome. They also showed that significant interaction of inflammation and metabolic system on cognition existed in the elderlies. After stratifying for inflammation, those with metabolic syndrome and high inflammation had an increased likelihood of cognitive impairment when compared to those without metabolic syndrome, while those with metabolic syndrome and low inflammation did not exhibit increased likelihood of impairment [3]. Other studies like Yaffe, Weston, Blackwill and Krueger [4], Gatto, Henderson, St John, c, Hodis and Mack Komulainen, Lakka, Kivipelto, Hassinen, Helkala, Haapala et al., [5], corroborated with the reports of Yaffe and colleagues [3].
Gottesman, Schneider, Albert, Alonso, Bandeen-Roche, Coker, Coresh and Co-workers [6], investigated midlife hypertension and 20-year cognitive change among African Americans and Caucasians. The cognitive change outcome included the 20-year change in scores on the Delayed Word Recall Test, Digit Symbol Substitution Test and Word Fluency Test as well as global cognition. Their findings show that baseline hypertension was associated with additional decline of 0.06 global cognition during 20 years and pre hypertension was associated non significantly with 0.04 global decline. Overall, they showed that midlife hypertension and elevated midlife but not late life systolic BP was associated with more cognitive decline during the 20 years of the study. Equally greater decline was found with higher midlife BP in white than in African Americans. There are extensive evidence emanating from studies of varying methodologies indicating that hypertension is associated with poorer cognitive performance on neuropsychological assessments including attention, learning and memory, executive functions and that clinical hypertension also predicts cognitive decline over time [7].
Investigations assessing the relationship between cholesterol level and cognitive functioning in healthy samples indicate that certain abilities may be inversely associated with serum cholesterol whereas others appear to be positively correlated with cholesterol concentration [8,9], evaluated associations between high density lipoprotein cholesterol (HDL) and non-HDL-C levels at specific ages and subsequent alzheimer disease (AD) risk. For non HDL-C, they found a significant association with AD risk in the 60 to 69 and 70 – 79 age bands, suggesting a potential U-shaped relationship (greater risk at low and high level). For example, in people aged 60 to 69, those with an average non HDL-C level of 120mg/DL had a 29% greater AD hazard than those with an average non HDL-C level of 160mg/DL, whereas those with an average non HDL-C level of 210mg/dl had a 16% greater hazard. However, Marcum and Colleagues (2018) did not find statistically significant association between HDL-C and AD risk. Similarly, Nooyens, Gelder, de-Mesquita, Boxtel and Verschuren [10], found that higher cholesterol intake was associated with faster cognitive decline, while higher n-z PUFA (especially a-lindenic acid) intakes was associated with slower decline in global cognitive function and memory as intakes of other fatty acids were not associated with cognitive decline. Sun, Lee, Ma and Kwok [11], had equally shown that among older people with diabetes mellitus, higher serum HDL-C was associated with better executive function. Also, Power and Colleagues (2018) showed that elevated total cholesterol, low density lipoprotein cholesterol and triglyceride were associated with greater 20-year decline in a test of executive function, sustained attention and processing speed.
On the other hand, blood sugar level has been associated with cognitive impairments in adult population. Altschul, Starr and Deary [12], assessed the correlation of cognitive function in early and later life and blood glucose in 1091 participants from Lothian Birth Control of 1936. Overall their findings showed high blood glucose to be consistently predicted by lower cognitive functions. Chaytor, Barbosa-Leiker, Ryan, Germine, Hirsch and Weinstock [13], showed that clinically significant cognitive impairment occurred in 48% of older adults with type 1 diabetes. After controlling for age, gender, education and diabetes duration, they showed that hypoglycemia unawareness, recent severe hypoglycemic events, microvascular complication, higher HbAIC and continuous glucose monitoring (CGM) average nocturnal glucose were all associated with increased odds of clinically significant cognitive impairment. However, CGM nocturnal % time below 60mg/dl was associated with a decreased odd of cognitive impairment. According to the authors, diabetes duration, age at diagnosis, daytime CGM and lifetime severe hypoglycemic events were not related to cognitive impairment status. Elfassy, Ajello, Schneiderman, Haan, Tarraf, Gonzalez, Gellman and colleagues [14], showed subgroup differences on relation of diabetes to cognitive function in Hispanics/Latinos of diverse backgrounds in the United States. Effassy and colleagues’ (2018) findings showed that compared with having normal glucose regulation, having diabetes was associated with worse processing speed among Cubans and Mexicans. As well compared with having normal glucose regulation, having pre-diabetes or diabetes was associated with worse delayed recall only among Mexicans. In essence, relationship between diabetes and cognitive function varied across Hispanic/Latino subgroups. Potentially, recent findings show strong relationships between blood glucose and cognitive decline in adult population. Kong, Park, Lee, Cho and Moon [15], showed increased insulin resistance to be significantly correlated with decreased cognitive function during a 6 year follow up study of older Koreans with normal baseline cognitive function.
Buch, Carmeli, Shefer, Keinan-Boker, Berner, Marcus, Goldsmith and Stern, [16], studied whether specific obesity phenotypes in community dwelling elderly affect differently the relationship between frailty and functional impairment and are equally related to cognitive impairment. Among other result outcomes, they showed that rate of cognitive impairment was 3.3 times higher in women who were obese by waist circumference but not by Body Mass Index (BMI). Mais, Georgiopoulos, Khan, Johnson, Wong, Charakida, Whincup et al., [17], studied patterns of adiposity, vascular phenotypes and cognitive function in the 1946 British Birth Cohort. Overall, their findings showed longer exposure to elevated waist circumference (WC) or BMI and faster WC or BMI gains between 36 and 43 years are related to lower cognitive function at 60-64 years. Equally, patterns of WC in adulthood could provide additional information in predicting late midlife cognitive function than patterns of BMI. Mangone, Yates, Sweat, Joseph and Convict [18], showed that executive function (EF) is negatively implicated in adolescents with mets. Additionally, waist circumference was determined to be significant predictor of the executive function (EF) deficits. Other studies like Bugge, Meller, Westfall, Tarp, Geji, Wedderkopp and Hillman [19], also supported the relationship between WC and other mets on cognitive function in adolescents.
Overall, findings from literature points to the contributions of mets and individual metabolic factors on cognitive performance in both adult and adolescent population. However little or no studies to the best of our knowledge have been done in Nigeria as regards to this. It is true that African American population have been studied to some extent, however, their findings cannot be readily extrapolated to African population. This problem of paucity of research of African origin limits global picture on causation/etiology of dementia. Equally general studies on mets have always considered global cognitive measures. Little effort has gone into evaluating the role of mets and anthropometry on processing speed. The present study therefore seeks to answer the following research question: (1) Do metabolic syndrome and its independent factors (BP, sugar level, total cholesterol, BMI) predict significantly cognitive performance (general cognition and processing speed) in mid adults from rural population in South-East Nigeria? (2) What are the contributions of some demographic variables (education, occupation, gender, age) and anthropometric measure (Height, weight) on cognitive performance in the same population? We hypothesise that metabolic syndrome, education and gender will significantly predict global cognitive performance and processing speed in the sample studied.
Method
Participants
The sample included 172 middle age adults from a rural community in Anambra State Nigeria. Their age range was between 50 – 70 years with a mean age of 57.48 and SD age of 5.45. Table 1 shows the descriptive statistics of the participants. For the present study, the young mid age were participants between 50-59 age while the old mid age were those between the ages of 60-70 years. The professional category are the participants with minimum of tertiary education who were engaged in professional jobs like teachers, counsellors, physicians. The non professional category for the present study were participants engaged in other forms of jobs like trading, hand craft and low level secretary and assistants. They were mostly with primary and secondary education.
Instruments
The Montreal Cognitive Assessment (MoCA) was used to assess general cognition among participants. MoCA developed by Nasreddine in 1996 [20], is a widely used screening assessment for detecting cognitive impairment. It has been validated in clinical settings assessing mild cognitive impairment and has subsequently been adopted in numerous other clinical settings. The Symbol Digit Modalities Test (SDMT) [21,22], was developed to identify individuals with neurological impairment. The SDMT assesses key neurocognitive functions that underlie many substitution tasks including attention, visual scanning and motor speed. The SDMT is an altered inverse form of Digit Symbol Test (DST) [23], equally used in the present study. Correlations of the SDMT with the Digit Symbol Test, test-retest correlations for the SDMT as well as the correlation between its written and oral administrations are all on the order of 0.80 for normal subjects [24,25]. For the study, the SDMT and DST were used to assess the participant’ processing speed.
The Body Mass Index (BMI) was calculated as the ratio of weight (in kilograms) to height (in meters) squared. Height and weight were measured objectively with a clinical calibrate scale. Waist circumference were obtained with a flexible tape measure, manipulated to maintain close contact with the skin without compression of underlying tissues. Waist circumference was defined as the minimal abdominal perimeter located half way between the rib cage and pelvic crest. The metabolic syndrome were got through the use automated diagnostic kits for blood sugar level and cholesterol level measurement. Equally, automated sphygmomanometer kit for blood pressure assessment was used to measure the blood pressure of the participants.
Procedure
The participants for the study were recruited through weekend door to door knocking in the community. This way, the research assistants were able to identify the participants within the given age bracket of the study interest. The houses were mapped for visit. The testing were done in the participants’ houses where they were more comfortable to respond. All the questionnaires and the neuropsychology tests were administered the same day for each participant with some breaks at intervals. The blood samples for the cholesterol and sugar analysis were equally taken at the end of the exercise. The research assistants explained to the participants that the study was to determine the contributions of some variables on cognitive functioning and solicited for their co-operations. They were all required to sign informed consent for the study which all complied. For few that were unable to read and understand the consent form, their relatives explained to them and then had to consent after they had understood the form.
Design and Statistic
The study was a community survey design. Multiple regression analysis and a 3-Way Multiple Analysis of Covariance (MANCOVA) statistics were used in analyzing the data. The regression analysis was used to determine the predictive strengths of the predictor variables (metabolic syndrome and anthropometry) on cognition, while the MANCOVA was used to analyse the test of between differences of the demographic variables on cognition. A pairwise comparisons was used as a post hoc analysis for significant demographic variable.
Results
Metabolic and Anthropometric Predictors of Cognition
Our analysis started with a stepwise regression of the cardiovascular markers of metabolic syndrome. The stepwise regression shows the systolic blood pressure (BP) as the only significant predictor of general cognition based on the MoCA, with standardized co-efficient β = 0.15; while the diastolic BP; β = 0.006 and Pulse: β = 0.001 all at P ≤ 0.05 level of testing. The overall model contribution (R2) was 0.02, while the ANOVA model was not significant at P < 0.05. The data show that the systolic blood pressure accounted for the overall contributions to the model. On the other hand, we entered other metabolic and anthropometric markers against MoCA and the findings show: BMI; β = 0.55, weight; β = 0.54 and waist circumference; β = 0.39 as significant predictors of general cognition. The sugar level; β = 0.20; total cholesterol; β = 0.18, and Height β = 0.003 were not significant predictors of general cognition.
The same variables were equally regressed for the digit symbol modality test (DSMT) as measure of processing speed. The Cardiovascular markers showed no significant predictions on the DSMT tasks and the ANOVA model fit was also not significant at P < 0.05. The overall contribution to the model (R2) was 0.04, while β scores for the individual variables were: systolic = 0.12; diastolic = 0.17 and pulse = 0.03. When the cardio markers were regressed on symbol digit test (SDT), the findings show an R2 of 0.11, while the ANOVA model fit was significant at P ≤ 0.002 level of testing. The standardized coefficients Symbol were systolic = 0.34; diastolic = 0.05 and pulse = 0.02. Only the systolic blood pressure had significant Symbol coefficient.
The other metabolic and anthropometric variables were also regressed in the model. The BMI, waist circumference, height and weight were first regressed on DSMT. Their overall model contribution was R2 = 0.05 and the ANOVA model fit showed no significance at P ≤ 0.05 level of testing. Equally the Symbol coefficients were not significant at P ≤ 0.05; BMI = 0.10, height = 0.12 and weight = 0.07. When regressed on SDT, the R2 was 0.14 while the ANOVA model fit was significant at P ≤ 0.002. The β coefficients had no significant predictions at P ≤ 0.05 except the waist circumference with Symbol = 0.20. The other Symbol coefficients were BMI = 0.17; Height = 0.18 and Weight = 0.03. The total cholesterol (TC) and sugar levels were also regressed on DSMT. As expected, their R2 was 0.53 though the ANOVA model was not significant at P ≤ 0.05. Their Symbolcoefficients were; sugar level = 0.52; total cholesterol = 0.31. However, the coefficients did not show significance at P ≤ 0.05 level of testing. On SDT, the total cholesterol and sugar level had the R2 of 0.66 while the ANOVA fit model was significant at P ≤ 0.02 level. The Symbol coefficients were sugar level = 0.65 (P ≤ 0.05) and cholesterol = 0.26 (P ≤ 0.34).
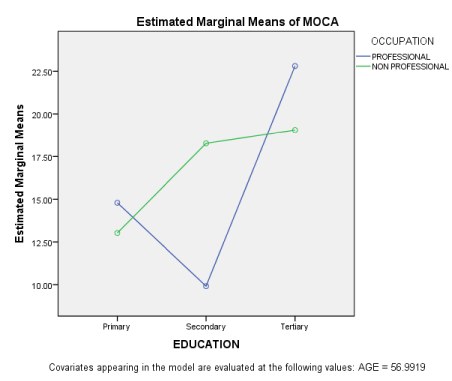
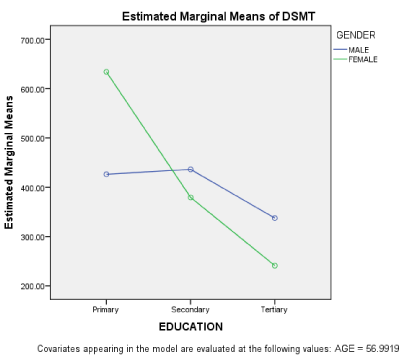
When age was regressed on MoCA, the finding shows little overall contribution of Age; R2 = 0.07, although the ANOVA model fit was significant at P ≤ 0.001. The Symbol coefficient was 0.27 at P ≤ 0.001. Education, occupation and gender were equally analysed as demographic variables using the 3 WAY MANCOVA with age a covariate. The findings show significant differences of education on the 3 measure of cognition studied: SDMT; F(2, 123) = 15.31; DSMT; F(2,123) = 8.95 and MoCA; F(2,123) = 5.32 all at P < 0.001 level. Their effect sizes were equally high ranging from 0.14 – 0.22. However, all other demographic variables showed no significant differences on cognition. The following were their MANCOVA measures; Occupation: SDMT, F(1, 123) = 0.59; DSMT, F (1, 123) = 0.98; MoCA, F(1, 123) = 0.04. Gender: SDMT, F(1, 123) = 0.01; DSMT, F (1, 123) = 1.27; MoCA, F(1, 123) = 0.42. Age as a covariate showed no significant difference. On the interaction effects, our findings show no interaction effect of the independent variables on cognition at P ≤ 0.05 except that of education* occupation on MoCA , F(2, 123) = 3.517, and education* gender on DSMT, F (2, 123) = 3.21, although with less effect sizes of 0.06 respectively. Table 2 shows the estimated marginal means of education on the dependent variables (cognition).
Table 1 above shows that individuals with primary and secondary school education took more time to complete the SDMT and DSMT and equally had less scores on MoCA compared to the tertiary education Table 3.
The pairwise comparison of table 2 shows that individuals with tertiary education significantly differed from the other two groups across the cognitive domains examined. The interaction effects are shown in the figures below.
Figure 1 shows the interaction effect of education and occupation on MoCA. The figure shows that an individual’s occupation interacts with education to influence performance on MoCA. From the graph, individual with high level of education and on professional occupation performs better than an individual with high level of education but on non- professional job. On the other hand, professionals with secondary education performs worse than non-professional with secondary education.
On figure 2, gender interacted with education on DSMT in females. From the graph, females with more education performed better than their counterparts with less education. But such interaction was only noticed in men on higher education.
We later added metabolic syndrome (mets) into the demographic model. Mets shows no significant differences on MoCA, SDMT and DSMT respectively; MoCA (F = 0.78, P ≤ 0.005, ES = 0.02); SDMT (F = 2.37, P ≤ 0.05, ES = 0.05); DSMT (F = 0.33, P ≤ 0.05; ES = 0.01). However significant differences were found on the interaction effect of mets and Education on MoCA, SDMT and DSMT. MoCA (F = 3.51, P ≤ 0.02, ES = 0.10), SDMT (F = 2.83, P ≤ 0.04, ES = 0.08), DSMT (F = 2.61, P ≤ 0.05, ES = 0.8). Due to the very poor effect size, pattern of their interactions were not reported. BMI categories were also analysed for MoCA because it shows high predictive strength. The result showed significant difference (F = 3.60, P ≤ 0.05, ES). Their men scores on MoCA were Normal BMI = 18.36; Overweight = 16.30 and Obese = 15.34.
Discussion
Our findings show that cardiovascular markers were not significant predictors of global cognitive impairment as measured with MoCA except the systolic blood pressure. The overall contribution of the model as well as the ANOVA fit were not encouraging. The impression was that cardiovascular markers assessed in the study (blood pressure) were not strong predictors of overall cognitive impairment in the sample studied. Blood pressure has always been implicated as a causal factor in cognitive impairment. However mixed findings have trailed this hypothesis. Some findings show that low blood pressure causes cognitive impairment while others show the opposite [26]. Some studies assessing the effect of late-life blood pressure levels reported that low diastolic and very high systolic levels may increase the risk [26], while midlife blood pressure have been reported consistently to show a harmful effect on late life cognition. Our findings show that blood pressure among adults between 50 – 70 years did not significantly predict general cognitive impairment and were supported by those of Herbert, Scherr, Bennet, Bienias, Wilson et al., [27], and those of Solfrizzi and Colleagues [28], that equally reported no significant effect of high blood pressure on cognition. Blood pressure may not directly cause cognitive impairment but through several mechanisms can potentially contribute to cognitive impairment. Reitz & Luchsinger, [26], reviewed three of such mechanisms. First they discussed about hypertension being a risk factor for ischemic cortical infarcts and subcortical white matter lesions which both play a role in cognitive impairment. Secondly, blood pressure may trigger or cause a blood-brain dysfunction which has been suggested to be involved in the etiology and pathogenesis of AD [29,30]. Another potential mechanism for association of blood pressure and cognition are shared risk factors such as the formation of free oxygen radicals [26], It may not necessary be the blood pressure that leads to cognitive impairment rather its contribution as a risk factor to vascular diseases. In line with this, McGuiness, Todd, Passmore and Bullock [31], found no convincing evidence from their study that blood pressure lowering prevents the development of dementia or cognitive impairment in hypertensive patient with no apparent prior cerebrovascular disease.
The BMI, weight and waist circumference from our study predicted significantly general cognitive impairment in the participants. One-Way ANOVA shows that obese participants performed poorer than the normal BMI and overweight counterparts. Gunstad and Colleagues (2010) using the Baltimore longitudinal study of aging show that BMI, waist circumference, waist to hip ratio as well as weight were associated with poorer global cognitive function test. As stated, obese sample from our study show significant poorer performance on general cognitive function. There are several explanations that might account for these findings other than the medical conditions that frequently comorbid with obesity including hypertension and type 2 diabetes Disorders like vascular pathology (e.g. endothelial dysfunction), reduced cardiovascular fitness, inflammatory process and neuroendocrine dysregulation are prevalent among obese individuals and are associated with poor neurocognitive function [32]. Surprisingly, our result showed direct relationship/prediction of weight and waist circumference on general cognitive function. Ordinarily, an inverse relationship was expected. Our study is in line with some studies that have suggested the roles of weight loss in dementia. For e.g., Steward and Colleagues [33] in the Honolulu – Asia ageing study showed that dementia associated weight loss begins before the onset of the clinical syndrome and accelerates by the time of diagnosis. Barrett–Connor and Co-Workers [34], in their 20 years longitudinal study of older community dwelling men and women showed that weight loss precedes mild to moderate dementia and that early weight loss is therefore unlikely to be a consequence of AD patients being unable or unwilling to eat. Further work is needed to better understand the association between weight and general cognitive function in mid and older adult populations.
The cardiovascular marker did not predict neural processing speed as measured by digit symbol coding tests. However, diastolic blood pressure had significant prediction on processing speed as measured by the Symbol Digit Test (SDT). Bueur and Madden [35], had reported no significant difference between chronically high blood pressure (BP) and normal blood pressure person on tests of processing speed even when race and years of education were added as covariates. Iadecoha and Colleagues [36], show that hypertension had insufficient data evidence-based recommendations for cognitive functions. Hypertension may be critical to cognitive decline by disrupting the structure and function of cerebral blood vessels leading to ischemic damage of white matter regions [36]. It appears that poor control of hypertension instead of hypertension may adversely account for cognitive decline. Thus, judicious treatment of hypertension taking into account goals of care and individual characteristics (e.g. age and comorbidities), seems justified to safeguard vascular health and as a consequence, brain health [36]. On the same note, BMI and other anthropometric measures (WC, Weight, Height) in this study did not predict significantly processing speed, although they were implicated on general cognitive function. Smith, Hay, Campbell and Trollor [37], reviewed 19 articles on association between BMI and cognitive functions in the adults. Their findings supported the contributions of BMI (obesity) on cognition but particularly the executive functions and working memory. Clark, Huiping, Callahan and Univerzagt [38], in their secondary analysis of multisite randomized trial on advanced cognitive training in the older adults showed that training effect on the reasoning and speed of processing outcomes did not differ by BMI status, although that on the memory outcome in the participants was 38% improvement in favour of normal weight BMI. Conversely, demographic variables only had education as major variable influencing cognition. The participants’ education differed significantly on processing speed. Education has been judged as part of factors in cognitive reserve and seems to delay onset of dementia [39-43]. The result of the present study supported past findings on the roles of education on cognitive performance.
Limitations of the Study
Our study was a survey research study and thus certain precautions that should be applied in clinical studies were not taken into consideration. For example, we did not put into consideration co-morbid vascular disorders while measuring blood pressure. Equally, we did not dichotomise the blood pressure into normal and abnormal blood pressures. This could have helped determine more the roles of blood pressure on cognition. Also, the number of participants for the study were limited. Some of the participants were not included in the total cholesterol and blood sugar level tests due to some logistics that prevented their inclusion. Further studies that can employ larger sample population could help more to validate the findings.
Conclusion
Our study shows that blood pressure as a cardiovascular marker do not predict significantly general cognitive performance as measured by the Montreal Cognitive Assessment test (MoCA). It also did not predict significantly the processing speed as measured by two gold standards for speed of processing measurement. However, some components of metabolic syndromes including the BMI, weight and waist circumference significantly predicted general cognitive functions as well as the processing speed. Differential effects of BMI were also seen on cognition with normal BMI showing better cognitive performance when compared to the obese and overweight.
- Kaur J (2014) A comprehensive, review on metabolic syndrome. Cardiol Res Pract 2014: 943162. Link: http://bit.ly/2ZQLRca
- Kathy F Yates, Victoria Sweat, Po Lai Yau, Michael M Turchiano, Antonio Convit (2012) Impact of metabolic syndrome on cognition and brain: A selected review of literature. Arteriosclerosis, Thromb Vasc Biol 32: 2060-2067. Link: http://bit.ly/2TppA2A
- Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, et al. (2004) The metabolic syndrome, inflamtion and risk of cognitive decline. JAMA 292: 2237-2242. Link: http://bit.ly/33r77HH
- Yaffe K, Weston AL, Blackwell T, Krueger KA (2009) The metabolic syndrome and development of cognitive impairment among older women. Arch Neurol 66: 324-328. Link: http://bit.ly/2McV8YU
- Komulainen P, Lakka TA, Kivipelto M, Hassinen M, Helkala EL, et al. (2007) Metabolic syndrome and cognitive function: A Population based follow up study in elderly women. Dement Geriatr Cogn Disord 23: 29-34. Link: http://bit.ly/2TuhQwr
- Gottesman RF, Schneider AL, Albert M, Alonso A, Bandeen-Roche K, et al. (2014) Midlife hypertension and 20 year cognitive change: The Atherosclerosis risk in communities neurocognitive study. JAMA Neurology 71: 1218-1227. Link: http://bit.ly/2MXNuRB
- Waldstein SR, Katzel LI (2001) Hypertension and cognitive function. In S.R. Waldstein and M.F. Elias (eds.), Neuropsychology of cardiovascular disease. Mahwah New Jersey: Lawrence Erlbaum Associates Publishers.
- Muldoon MF, Flory JD, Ryan CM (2001) Serum Cholesterol, the brain and cognitive functions. In S.R. Waldstein and M.F. Elias (eds.), Neuropsychology of cardiovascular disease. Mahwah, New Jersey: Lawrence Erlbaun Associates Publishers.
- Marcum ZA, Walker R, Bobb JF, Sin MK, Gray SL, et al. (2018) Serum Cholesterol and incident Alzheimer’s diseases: Findings from the adult changes in thought study. J Am Geriatr Soc 66: 2344-2352. Link: http://bit.ly/2Mf69c4
- Nooyens ACJ, van Gelder BM, Bueno-de-Mesquita HB, van Boxtel MPJ, Verschuren WMM (2018) Fish consumption, intake of fats and cognitive decline at middle and older age: The Doetinchem cohort study. Eur J Nutr 57: 1667-1675. Link: http://bit.ly/2MUXRFW
- Sun Y, Lee J, Ma RC, Kwok T (2018) Serum high density lipoprotein cholesterol is a predictive predictor of executive function in older patients with diabetes mellitus. J Diabetes Investig 10: 139-146. Link: http://bit.ly/2ZVyEyN
- Altschul DM, Starr JM, Deary IJ (2018) Cognitive function in early and later life is associated with blood glucose in older individuals: analysis of the Lothian Birth Control of 1936. Diabetologia 61: 1946-1955. Link: http://bit.ly/2YHyZZf
- Chaytor NS, Barbosa-Leiker C, Ryan CM, Germine LT, Hirsch IB, et al. (2019) Clinically significant cognitive impairment in older adults with type 1 diabetes. J Diabetes Complications 33: 91-97. Link: http://bit.ly/2Tqxp8d
- Elfassy T, Aiello AE, Schneiderman N, Haan MN, Tarraf W, et al, (2018) Relation of diabetes to cognitive function in Hispanics/Latinos of diverse backgrounds in the United States. J Aging Health 31: 1155-1171. Link: http://bit.ly/2YSljKx
- Kong SH, Park YJ, Lee JY, Cho NH, Moon MK (2018) Insulin resistance is associated with cognitive decline among older Koreans with normal baseline cognitive function: A prospective community based cohort study. Sci Rep 8: 650. Link: http://bit.ly/2KuFXs7
- Buch A, Carmeli E, Shefer G, Keinan-Boker L, Berner Y, et al. (2018) Cognitive impairment and association between frailty and functional deficits are linked to abdominal obesity in the elderly. Maturitas 114: 46-53. Link: http://bit.ly/2YYBe9Z
- Masi S, Georgiopoulos G, Khan T, Johnson W, Wong A, et al. (2018) Pattern of adiposity, vascular phenotype and cognitive function in 1946 British Birth Control. BMC Medicine 16: 75. Link: http://bit.ly/2TrzOQf
- Mangone A, Yates KF, Sweat V, Joseph A, Convit A (2018) Cognitive functions among predominantly minority urban adolescents with metabolic syndrome. Appl Neuropsychol Child 7: 157-163. Link: http://bit.ly/2Krv1LH
- Bugge A, Moller S, Westfall DR, Tarp J, Geji AK, et al. (2018) Association between waist circumference, metabolic risk and executive function in adolescents: A cross sectional mediation analysis. PLOS ONE 13: e0199281. Link: http://bit.ly/2H1Y3zD
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, et al. (2005) The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53: 695-699. Link: http://bit.ly/2Z6q5Eo
- Smith A (1968) The Symbol Digit Modalities Test: A neuropsychological test of learning and other cerebral disorders. In J Helmuth (ed.), Learning disorders. Scatlle: Special Child Publications.
- Smith A (1982) Symbol Digit Modalities Test. Los Angeles: Western Psychological Service.
- Wechsler D (1955) Manual for the Wechsler Adult Intelligence Scale (WAIS). New York: The Psychological Corporation
- Lezak MD, Howieson DB, Loring DW, Hannay HJ, Fischer JS (2004) Neuropsychological Assessment (4th Ed). New York: Oxford University Press. Link: http://bit.ly/31BuNrh
- Spreen O, Straus E (1998) A Compendium of neuropsychological tests (2nd ed.). New York: Oxford University Press. Link: http://bit.ly/2MemRZ8
- Reitz C, Luchsinger JA (2007) Relation of blood pressure to cognitive impairment and dementia. Curr Hypertens Rev 3: 166-176. Link: http://bit.ly/33uLvdl
- Herbert LE, Scherr PA, Benneth DA, Bienias JL, Wilson RS, et al. (2004) Blood pressure and late life cognitive function change a bi-racial longitudinal study. Neurology 62: 2021-2014. Link: http://bit.ly/2YWj4pA
- Solfrizzi V, Panza F, Colacicco AM, D’Introno A, Capurso C, et al. (2004) Vascular risk factors, incidence of MCI and rates of progression to dementia. Neurology 63: 1882-1891. Link: http://bit.ly/2KIbqps
- Skoog I (1997) The relationship between blood pressure and dementia: A review. Biomed Pharmacother 51: 367-375. Link: http://bit.ly/33rDczd
- Skoog I, Walin A, Fredman P, Hesse C, Aevarsson D, et al. (1998) A population study on blood-brain barrier function in 85-year-olds Relation to Alzheimer's disease and vascular dementia. Neurology, 50: 966-971. Link: http://bit.ly/2MQqYdA
- McGuiness B, Todd S, Passmore P, Bullock R (2006) The effects of blood pressure lowering on development of cognitive impairment and dementia in patients without apparent prior cerebrovascular disease. Cochrane Database Syst Rev 19: CD004034. Link: http://bit.ly/2ZVbDvY
- Sapolsky RM (1999) Glucocorticoids, stress and their adverse neurological effects: relevance to aging. Exp Gerontol 34: 721-732. Link: http://bit.ly/33nfsMF
- Stewart R, Masaki K, Xue QL, Peila R, Petrovitch H, et al. (2005) A 32 year prospective study of change in body weight and incident dementia. The Honolulu–Asia Aging study. Arch Neurol 62: 55-60. Link: http://bit.ly/2N0XVUR
- Barrett-Connor E, Edelsterin SL, Corey-Bloom J, Wiederholt WC (1996) Weight loss precedes dementia in community dwelling older adults. J Am Geriatr Soc 44: 1147–1152. Link: http://bit.ly/2MWZMKk
- Barbara Bucur, David J Maddena (2010) Effects of adult age and blood pressure on executive function and speed of processing. Exp Aging Res 36: 153-168. Link: http://bit.ly/2MbMOJ5
- Iadecola C, Yaffe K, Biller J, Bratzke LC, Faraci FM, et al. (2016) Impact of Hypertension on Cognitive Function: A Scientific Statement From the American Heart Association. Hypertension 68: e67-e94. Link: http://bit.ly/2McGz7Q
- Smith E, Hay P, Campbell L, Trolbor JN (2011) A review of the association between obesity and cognitive functions across the life span: Implications for novel approaches to prevention and treatment. Obes Rev 12: 740-755 Link: http://bit.ly/2ZUhWjq
- Clark DO, Xu H, Callahan CM, Unverzagt FW (2016) Does body mass index modify memory, reasoning and speed of processing training effects in older adults. Obesity 24: 2319-2326 Link: http://bit.ly/33rf1kq
- Luerding R, Gebel S, Gebel EM, Schwab-Malek S, Weissert R (2016) Influence of formal education on cognitive research in patients with multiple sclerosis. Front Neurol 7: 46. Link: http://bit.ly/2yUXmmZ
- Le Carret N, Lafont S, Mayo W, Fabrigoule C (2003) The effect of education on cognitive performance and its implication for the constitution of the cognitive research. Dev Neuropsychol 23: 317-337 Link: http://bit.ly/2Kv6XaY
- Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, et al. (2005) Aggregation of vascular risk factors and risk of incident alzheimer disease. Neurology 65: 545-551. Link: http://bit.ly/2YF3UFH
- Power MC, Rawlings A, Sharrett AR, Bandeen-Roche K, Coresh J, et al. (2018) Association of midlife lipids with 20 year cognitive change: A cohort study. Alzheimers Dement 14: 167-177. Link: http://bit.ly/2McRj63
- Ylikoski R, Ylikoski A, Raininko R, Keskivaara P, Sulkava R, et al. (2000) Cardiovascular diseases, health status, brain imaging findings and neuropsychological functioning in neurologically healthy elderly individuals. Arch Gerontol Geriatr 30: 115-130. Link: http://bit.ly/2KsaKps

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
